Abstract
Bile duct stones, indeterminate biliary strictures and other biliary duct pathologies represent a significant surgical and endoscopic challenge in patients with altered luminal or biliary anatomy. Traditional endoscopic retrograde cholangiopancreatography (ERCP) is not feasible and alternative approach is usually required. A novel alternative approach of addressing these challenging cases is assessed by this case series. All patients who underwent percutaneous transhepatic cholangioscopy (PTCS) and SpyglassTM Direct visualization system (SDVS) between December 2016 and February 2018 were studied. The indications for procedure, interventions performed, outcomes and complications were reviewed for each case. SpyglassTM marketed by Boston Scientific Corporation, Marlborough, Massachusetts was utilized by interventional endoscopists and radiologists through a 12 French (Fr) percutaneous vascular sheath. Five patients had altered biliary and/or luminal anatomy: two with Roux-en-Y gastric bypass and three with Roux-en-Y hepaticojejunostomy. All patients had unsuccessful previous ERCP attempts. All PTCS with SDVS procedures were technically successful. Indications for this unusual approach were: ascending cholangitis, abnormal liver function tests and biliary dilation on imaging. SDVS was utilized to conduct electrohydraulic lithotripsy (EHL) for biliary stone management in four patients and intraductal biopsies for indeterminate strictures in two of them. PTCS with SDVS can be beneficial for multiple diagnostic and therapeutic indications in patients with altered biliary or luminal anatomy. SDVS allows direct evaluation and management of different biliary pathologies in challenging cases where traditional ERCP is not feasible. Some indications for PTCS with SDVS include evaluation of biliary strictures and biliary stasis, biliary tract biopsy and lithotripsy for management of biliary stones.
Keywords: Cholangioscopy, percutaneous transhepatic cholangioscopy (PTCS), Spyglass, case series
Introduction
Patients with altered luminal anatomy who require biliary drainage, sampling or other diagnostic and therapeutic interventions may not be suitable for the traditional endoscopic retrograde cholangiopancreatography (ERCP) (1,2). Methods to endoscopically access the remnant stomach, including enteroscopy-assisted ERCP or laparoscopy-assisted ERCP in gastric bypass patients (3), and/or endoscopic ultrasound guided gastro-gastric fistula creation (4), have been described with variable success rate in the literature. We hereby describe an alternative approach that allows access to the biliary tree with direct visualization and sampling of the bile duct via cholangioscopy; this novel approach additionally facilitates optically guided intraductal fragmentation and clearance of biliary calculi (1,5). This case series identifies five patients who successfully underwent percutaneous transhepatic cholangioscopy (PTCS) with SpyglassTM Direct visualization system (SpyglassTM DS Boston Scientific Corporation, Marlborough, Massachusetts). Since SpyglassTM scope requires at least a 12 French (Fr) percutaneous tract, the initial 8 Fr percutaneous transhepatic biliary drainage (PTBD) catheter was upsized to 12 Fr and the bile duct was explored endoscopically via a standard 12 Fr percutaneous vascular sheath once the tract matured. We present the following case series in accordance with the CARE-Guideline (6).
Patient case 1
A 59-year-old male with liver transplant with Roux-en-Y hepaticojejunostomy in 2008 and re-transplant in 2015 for chronic rejection presented with fever of 38.6 °C. He was found to have obstructive pattern liver enzymes; blood cultures during the admission were positive for Escherichia coli bacteremia, and diagnosis of cholangitis was made. Magnetic resonance cholangiopancreatography (MRCP) revealed anastomotic stricture at hepaticojejunostomy and choledocholithiasis at allograft bile duct. ERCP was not attempted due to the altered anatomy. Percutaneous transhepatic cholangiography (PTCS) revealed marked dilatation of the common bile duct (CBD) with large stones. PTCS was performed with the SpyglassTM introduced and advanced to the lower third of the main duct. The lower third of the main bile duct and middle third of the main bile duct contained two stones, the larger of which was 10 mm in diameter. EHL was performed successfully and hepaticojejunostomy anastomosis was visualized and crossed with SpyglassTM without difficulty. Using balloon sweep, the stones were swept from the bile duct into the anastomosis by interventional radiology (IR); post-procedure cholangioscopy demonstrated a patent anastomosis with resolution of large indwelling stones. Eleven weeks later, over the wire PTCS revealed no filling defect. However, a stenosis involving hepaticojejunostomy resulting in markedly slow flow of contrast to the bowel, which improved with 14 Fr placement, was identified. In the subsequent two months, a cholangiogram through the existing PTBD catheter demonstrated anastomotic stricture despite a patent anastomosis. Stricture cholangioplasty was performed with a new over the wire 14 Fr internal/external biliary drainage catheter placement. A cholangiogram, ten weeks later, did not reveal any filling defects as the contrast was observed freely draining into the bowel from the biliary tree; thus, the PTBD catheter was removed. Patient experienced recurrent cholangitis until hepaticojejunostomy stricture was corrected; thereafter, no further symptoms were present on follow-up.
Patient case 2
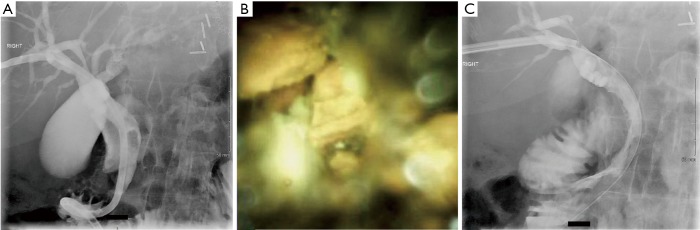
A 74-year-old male with Roux-en-Y gastric bypass presented with severe acute cholangitis and hyperbilirubinemia due to choledocholithiasis. Due to altered anatomy and urgent need for biliary decompression, the patient had a temporizing 8 Fr internal-external PTBD catheter placed. A month later, after upsizing the PTBD catheter to 12 Fr, the SpyglassTM was advanced percutaneously to the lower third of the main duct. The upper third of the CBD contained three stones as revealed in Figure 1A, which were successfully fragmented via electrohydraulic lithotripsy (EHL). Duct clearance was achieved by balloon sweep performed by IR per Figure 1B. A few weeks later, a cholangiogram performed through the existing drainage catheter demonstrated no filling defects within the CBD or the remaining biliary tree as seen in Figure 1C. Subsequently, the percutaneous biliary drain was removed.
Figure 1.
Successful fragmentation and removal of common bile duct stones using spyglass and EHL. (A) Initial percutaneous transhepatic cholangiogram and drain showed dilated common bile duct and filing defects; (B) common bile duct stones fragmented under direct visualization using spyglass and EHL; (C) post EHL and balloon sweep, no filling defects within the biliary tree. EHL, electrohydraulic lithotripsy.
Patient case 3
A 52-year-old male with hepatitis C and alcoholic liver cirrhosis status post orthotopic liver transplant (OLT) in 2006 presented with persistent jaundice, pruritus and weakness. Patient had been admitted with severe biliary stricture a year earlier and was being managed with PTBD; however, he failed to follow-up after the biliary drainage catheter accidentally dislodged. MRCP revealed choledochojejunostomy post OLT, mild intrahepatic biliary ductal dilatation, and 20 mm hypointense filling defects in the common hepatic duct just proximal to the anastomosis likely representing an intraductal calculus versus a biliary polyp per Figure 2. Given recurrent biliary obstruction, a PTBD catheter was placed to facilitate SDVS. The cholangioscope was inserted via a percutaneous 12 Fr vascular sheath into the distal CBD. The bile duct was explored endoscopically. Evidence of a previous surgical anastomosis was seen in the middle third of the main bile duct. Mild erythema at this site was considered to reveal granulation tissue and biopsies were obtained for confirmation. Biopsy revealed small bowel mucosa with patchy chronic inflammation without evidence of malignancy. EHL was performed for stone fragmentations. Follow up cholangiogram demonstrated contrast freely draining from biliary system into small bowel; thus, the biliary drainage catheter was removed. Patient responded well at twelve months follow-up post-PTCS with SDVS.
Figure 2.
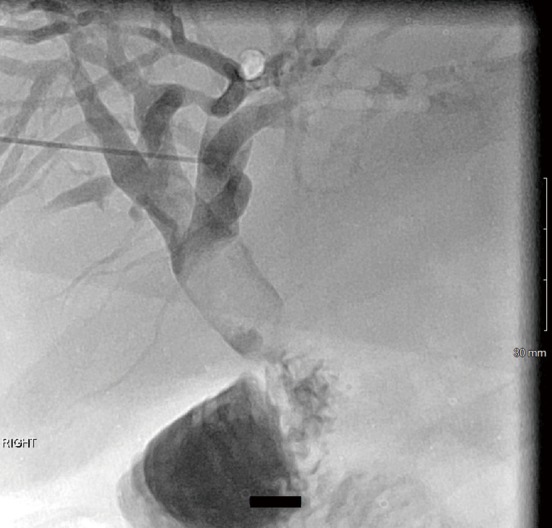
Percutaneous cholangiogram showed filling defect within the common hepatic duct proximal to the hepaticojejunostomy anastomosis.
Patient case 4
A 78-year-old male with an eight-year history of adenocarcinoma of gallbladder status post chemo-radiation, laparoscopic cholecystectomy, segmental resection of liver, gallbladder and extrahepatic bile duct resection and Roux-en-Y hepaticojejunostomy presented with six-week history of abdominal pain and twenty-pound weight loss. Imaging studies including contrast enhanced computed tomography (CT) abdomen and pelvis, and MRCP revealed ill-defined 25 mm low-density lesion at the hilum with moderate upstream biliary ductal dilation. Attempts at ERCP failed to reach the Roux limb. Internal-external PTBD catheter was placed and percutaneous cholangioscopy evaluation with SDVS was performed. This showed abnormal mucosa with prominent mucosal vessels and erythema at the hepaticojejunostomy anastomosis indicating presence of stricture. Biopsies were sent for histology and fluorescence in situ hybridization (FISH) testing, which revealed fragments of markedly inflamed and reactive mucosa with marked crush artifact; however, no tumor was identified and FISH testing resulted negative. Once biliary stasis resolved; the drainage catheter was removed with no consequences. Patient showed no evidence of obstruction twelve months post-PTCS.
Patient case 5
A 54-year-old female with factor II deficiency on warfarin, portal vein thrombosis, recurrent liver enzyme elevations, and Roux-en-Y gastric bypass presented with obstructive pattern liver enzymes. CT and MRCP revealed cholelithiasis and intrahepatic biliary ductal dilatation with CBD of up to 11 mm and a small obstructive CBD stone. PTCS demonstrated filling defect at the proximal CBD measuring about 5 mm by 9 mm, compatible with choledocholithiasis as seen in Figure 3. Internal-external PTBD was placed and subsequently the bile duct was explored with the SpyglassTM via PTCS. The main duct contained multiple stones. EHL fragmented the biliary stones and multiple sweeps of the CBD were performed to clear debris and stones. She did well on follow up with no further symptoms and was referred for cholecystectomy.
Figure 3.
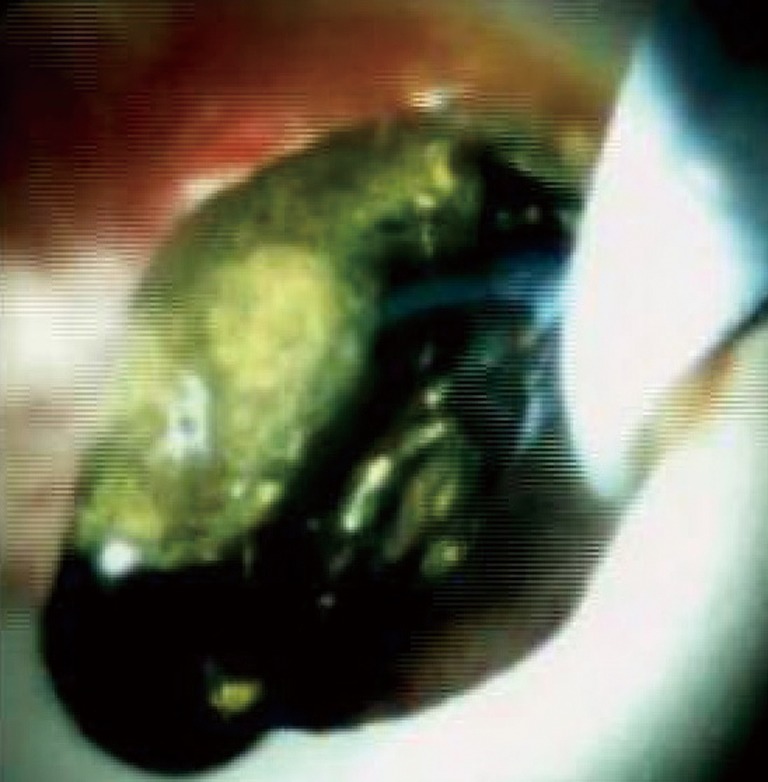
Choledocholithiasis revealed by SDVS. SDVS, SpyglassTM Direct visualization system.
Table 1 summarizes SDVS utility, findings and outcomes associated with all five cases reported in this case study.
Table 1. Utility, findings, and outcomes of percutaneous transhepatic cholangioscopy with SDVS.
| Case | Age sex | Presentation | Imaging findings | SDVS utility | SDVS indication | SDVS findings | Outcome | Complications | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 M | Fever, cholangitis | Post-transplant anastomotic stricture and choledocholithiasis | Therapeutic | Liver transplant with hepaticojejunostomy | Choledocholithiasis | EHL and removal of biliary stones | Recurrent cholangitis‡ | 27 |
| 2 | 74 M | Abdominal pain | A 13 mm dilated CBD at porta hepatis | Therapeutic | Roux-en Y gastric bypass | Three stones at upper third of CBD | EHL and removal of biliary stones | None | 21 |
| 3 | 52 M | Pruritus, jaundice | Diffuse narrowing of upper main bile duct and mild central intrahepatic biliary ductal dilatation | Diagnostic | Liver transplant with severe stricture in the upper third of CBD | Erythema at the hepaticojejunostomy site | Debris removal, Biopsy revealed small bowel mucosa with chronic inflammation | None | 12 |
| 4 | 78 M | Abdominal pain | Intrahepatic bile duct dilatation | Therapeutic & diagnostic | Hepaticojejunostomy post-hepatic resection for gall bladder carcinoma | Bile duct stones, abnormal mucosa at anastomosis | Persistent biliary ductal dilatation requiring prolonged PTBD | None | 12 |
| 5 | 54 F | Elevated liver enzymes | Intrahepatic and CBD dilatation, choledocholithiasis | Therapeutic & diagnostic | Roux-en-Y gastric bypass | Multiple bile duct stones and bile duct scarring | EHL and removal of biliary stones and biopsy | None | 19 |
‡, recurrent cholangitis was not a procedural complication but rather resulted from hepaticojejunostomy stricture. SDVS, SpyglassTM Direct visualization system; CBD, common bile duct; PTBD, percutaneous transhepatic biliary drainage; EHL, electrohydraulic lithotripsy.
Discussion
In mixed restrictive-malabsorptive bariatric procedures (gastric bypass and biliopancreatic diversion), the anatomy reaching the biliary system is significantly altered. Nonetheless, ERCP is challenging yet possible after gastric bypass either as EUS directed transgastric ERCP (EDGE) or laparoscopic assisted enteroscopy; on the other hand, following biliopancreatic diversion, the presence of interposed intestinal segment renders ERCP impossible (3,4). Bariatric surgeries, in addition to obesity, pose significant risk of cholelithiasis and choledocholithiasis (7). In these high risk and challenging patients, PTBD catheter offers a valuable access for SpyglassTM technique (2,8). PTCS with SDVS is described in Table 2.
Table 2. Description of the percutaneous transhepatic cholangioscopy with SpyglassTM Direct visualization system (SDVS).
| After obtaining informed consent, the patient is placed supine on the fluoroscopy table. The upper abdomen is prepared and draped in the usual sterile fashion using 2% chlorhexidine. After maintaining full sterile procedure, the skin overlying the right hepatic lobe is anesthetized with 5 cc of 2% lidocaine without epinephrine. A 21-gauze needle with stylet is then advanced into the liver and into the right biliary system under fluoroscopic guidance. Then, a percutaneous cholangiogram is performed. Next, a 0.018-inch mandril guide wire is introduced through the 21-gauze needle and advanced under fluoroscopic guidance into the central bile ducts. The 21-gauze needle is then exchanged for a 6 French coaxial system over indwelling 0.018-inch wire. Next, a 0.035-inch Amplatz wire is passed through the indwelling 6 French coaxial system and advanced into the small bowel under fluoroscopic guidance. The 0.018-inch wire is secured as a safety wire. The skin tract is dilated with an 8 French dilator. Next, the 6-French coaxial system is exchanged over the 0.018-inch wire for an 8-French multi-side hole external/internal biliary drain, which is advanced transhepatically over the Amplatz wire under fluoroscopic guidance until its tip is within small bowel and proximal side holes above the obstruction. Contrast injection is performed to confirm proper catheter position with side holes seen proximally and distally to the obstruction. The 8-French multi-side hole external/internal biliary drain’s pigtail is then formed, and the drain is anchored to the skin with a Roman sandal suture using 2-0 Prolene. The insertion site of the new internal-external biliary drain is then dressed with dry, sterile gauze and a Tegaderm, and the drain is connected to a drainage bag. Continuous catheter drain via the gravity drainage to the collection bag is performed for two days; the catheter can then be capped for internal drainage only |
| Depending upon the indication, if SpyglassTM procedure is necessary, the existing internal-external biliary drainage catheter is upsized by advancing 0.035-inch Amplatz wire through the pre-existing 8 French biliary drainage catheter. Next, the existing internal-external drainage catheter is removed. A 10 French dilator is inserted; then, internal-external biliary drainage catheter of appropriate size is advanced. Next, wire is removed, and pigtail is locked and formed. Repeat tube cholangiogram is performed to confirm the placement of the catheter. The internal-external drain is replaced approximately every 8 to 10 weeks. Once the underlying indication is treated, the internal-external biliary drainage catheter is replaced with an external biliary drainage catheter and capped for a week as a capping trial. If no symptoms of abdominal pain, fever or worsening liver enzymes exist, the drainage catheter may be ultimately removed |
Post liver transplant biliary complications represent another indication for endoscopic interventions (9). Increase in incidence of liver transplants along with successfully prolonged survival post-transplant has subsequently increased prevalence of transplant cases. Appropriateness of specific endoscopic intervention varies by the type of biliary reconstruction following transplant. In choledocho-choledochostomy, ERCP may be performed; however, in duct-to-duct anastomosis and Roux-en-Y, hepaticojejunostomy or choledochojejunostomy percutaneous cholangioscopy is required (9,10,11).
Accurate diagnosis of biliary strictures is similarly enhanced with the use of SpyglassTM cholangioscopy as it provides improved visibility and biopsy capabilities (12). Moreover, SpyglassTM cholangioscopy is valuable for clearance of biliary ductal stones not effectively removed by traditional maneuvers (4).
Compared to ERCP, PTCS is invasive, time consuming and painful; nonetheless, it offers a less invasive alternative to open or laparoscopic surgeries, which are associated with greater complication risks (13). SDVS offers direct visualization, and ability to obtain targeted biopsies and EHL under direct visualization (12). Moreover, several advantages of SDVS over traditional PTCS include: four-way steerability allowing improved maneuverability of the Spyglass, availability of independent irrigation channels to maintain clear cholangioscopic field, and single endoscopist operation (14,15). On the other hand, some shortcomings of SDVS include requirement of tract maturation prior to use, and fiber optic image quality inferior to ERCP video images (14). While the overall stone removal success rate using SDVS exceeds 90%, overall complication rate approaches only 5%, commonly including adverse events such as liver laceration, intra-abdominal abscess, cholangitis, acute pancreatitis, hemobilia, bile duct perforation, disruption of the PTBD fistula and septic shock (5,8). These complications can further be minimized by allowing PTCS tracts to mature for at least 2 weeks and gradually dilating it prior to SDVS (13). Additional limitations of SDVS include contraindication in active cholangitis to minimize intrahepatic abscess, bacteremia and sepsis; differing levels of SDVS operator experience and expertise may significantly result in outcome variability; moreover, PTBD tube must be maintained in place throughout the treatment period (1,2,5).
In conclusion, PTCS can be beneficial for multiple diagnostic and therapeutic indications especially in patients with altered surgical anatomy. Some indications for PTCS include evaluation of biliary strictures (benign or malignant), biliary tract biopsy, lithotripsy and removal of biliary stones, and undiagnosed biliary stasis evaluation.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No patient identifiers. Verbal informed consent obtained from patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nakai Y, Kogure H, Yamada A, et al. Endoscopic management of bile duct stones in patients with surgically altered anatomy. Dig Endosc 2018;30 Suppl 1:67-74. 10.1111/den.13022 [DOI] [PubMed] [Google Scholar]
- 2.Du L, D'Souza P, Thiesen A, et al. Percutaneous transhepatic cholangioscopy for indeterminate biliary strictures using the SpyGlass system: a case series. Endoscopy 2015;47:1054-6. 10.1055/s-0034-1392527 [DOI] [PubMed] [Google Scholar]
- 3.Abbas AM, Strong AT, Diehl DL, et al. Multicenter evaluation of the clinical utility of laparoscopy-assisted ERCP in patients with Roux-en-Y gastric bypass. Gastrointest Endosc 2018;87:1031-9. 10.1016/j.gie.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 4.Kedia P, Kumta NA, Widmer J, et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: a novel technique. Endoscopy 2015;47:159-63. 10.1055/s-0034-1390771 [DOI] [PubMed] [Google Scholar]
- 5.Laleman W, Verraes K, Van Steenbergen W, et al. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc 2017;31:2223-32. 10.1007/s00464-016-5221-2 [DOI] [PubMed] [Google Scholar]
- 6.Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. 10.1016/j.jclinepi.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 7.Li VK, Pulido N, Fajnwaks P, et al. Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc 2009;23:1640-4. 10.1007/s00464-008-0204-6 [DOI] [PubMed] [Google Scholar]
- 8.Franzini T, Cardarelli-Leite L, Figueira ERR, et al. SpyGlass percutaneous transhepatic cholangioscopy-guided lithotripsy of a large intrahepatic stone. Endoscopy 2017;49:E292-3. 10.1055/s-0043-117943 [DOI] [PubMed] [Google Scholar]
- 9.Hüsing-Kabar A, Heinzow HS, Schmidt HH, et al. Single-operator cholangioscopy for biliary complications in liver transplant recipients. World J Gastroenterol 2017;23:4064-71. 10.3748/wjg.v23.i22.4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arain MA, Attam R, Freeman ML. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl 2013;19:482-98. 10.1002/lt.23624 [DOI] [PubMed] [Google Scholar]
- 11.Girotra M, Soota K, Klair JS, et al. Endoscopic management of post-liver transplant biliary complications. World J Gastrointest Endosc 2015;7:446-59. 10.4253/wjge.v7.i5.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draganov P. The SpyGlass® Direct Visualization System for Cholangioscopy. Gastroenterol Hepatol (N Y) 2008;4:469-70. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Huang M, Yang J, et al. Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc 2005;19:505-9. 10.1007/s00464-004-8125-5 [DOI] [PubMed] [Google Scholar]
- 14.Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization,and biopsy. Gastrointest Endosc 2007;65:303-11. 10.1016/j.gie.2006.07.048 [DOI] [PubMed] [Google Scholar]
- 15.Yasuda I, Itoi T. Recent advances in endoscopic management of difficult bile duct stones. Dig Endosc 2013;25:376-85. 10.1111/den.12118 [DOI] [PubMed] [Google Scholar]