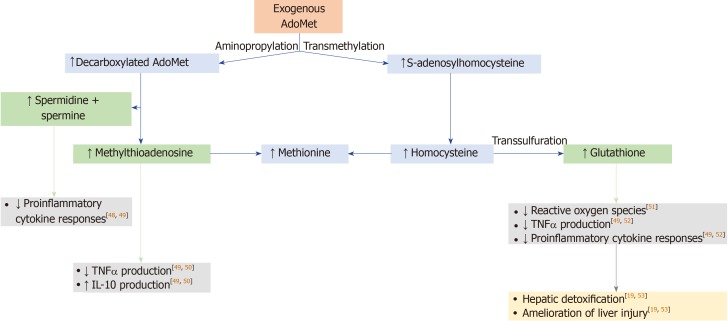
Figure 1.
Mechanism of action of S-adenosylmethionine[11,13,47]. S-adenosylmethionine (AdoMet)-dependent methylation reactions yield S-adenosylhomocysteine as a by-product, and S-adenosylhomocysteine is cleaved into adenosine and homocysteine by S-adenosylhomocysteine hydrolase. Remethylation of homocysteine to form methionine occurs by methionine synthase and betaine homocysteine methyltransferase. In the transsulfuration pathway, homocysteine is converted to cysteine, which is the rate-limiting precursor for glutathione, via an enzymatic process catalyzed by cystathionine β-synthase and cystathionase. To synthesize polyamines, AdoMet is decarboxylated in a reaction catalyzed by AdoMet decarboxylase. The predominant polyamines in mammalian cells are spermidine and spermine, which are made by sequential addition of aminopropyl groups from AdoMet decarboxylase; methylthioadenosine is a by-product of these reactions. These metabolites of AdoMet have biological effects[48-52], which may improve hepatic detoxification and amelioration of liver injury[19,53]. AdoMet: S-adenosylmethionine; IL: Interleukin; TNF: Tumor necrosis factor.