Abstract
Background
Faecal incontinence is a debilitating problem with significant medical, social and economic implications. Treatment options include conservative, non‐operative interventions (for example pelvic floor muscle training, biofeedback, drugs) and surgical procedures. A surgical procedure may be aimed at correcting an obvious mechanical defect, or augmenting a functionally deficient but structurally intact sphincter complex.
Objectives
To assess the effects of surgical techniques for the treatment of faecal incontinence in adults who do not have rectal prolapse. Our aim was firstly to compare surgical management with non‐surgical management and secondly, to compare the various surgical techniques.
Search methods
Electronic searches of the Cochrane Incontinence Group Specialised Register (searched 6 March 2013), the Cochrane Colorectal Cancer Group Specialised Register (searched 6 March 2013), CENTRAL (2013, issue 1) and EMBASE (1 January 1998 to 6 March 2013) were undertaken. The British Journal of Surgery (1 January 1995 to 6 March 2013), Colorectal Diseases (1 January 2000 to 6 March 2013) and the Diseases of the Colon and Rectum (1 January 1995 to 6 March 2013) were specifically handsearched. The proceedings of the Association of Coloproctology of Great Britain and Ireland annual meetings held from 1999 to 2012 were perused. Reference lists of all relevant articles were searched for further trials.
Selection criteria
All randomised or quasi‐randomised trials of surgery in the management of adult faecal incontinence (other than surgery for rectal prolapse).
Data collection and analysis
Three review authors independently selected studies from the literature, assessed the methodological quality of eligible trials and extracted data. The three primary outcome measures were change or deterioration in incontinence, failure to achieve full continence, and the presence of faecal urgency.
Main results
Nine trials were included with a total sample size of 264 participants. Two trials included a group managed non‐surgically. One trial compared levatorplasty with anal plug electrostimulation and one compared an artificial bowel sphincter with best supportive care. The artificial bowel sphincter resulted in significant improvements in at least one primary outcome but the numbers were small. The other trial showed no difference in the primary outcome measures.
Seven trials compared different surgical interventions. These included anterior levatorplasty versus postanal repair, anterior levatorplasty versus total pelvic floor repair, total pelvic floor versus postanal repair, end to end versus overlap sphincter repair, overlap repair with or without a defunctioning stoma or with or without biofeedback, and total pelvic floor repair versus repair plus internal sphincter plication and neosphincter formation versus total pelvic floor repair. Sacral nerve stimulation and injectables are considered in separate Cochrane reviews. Only one comparison had more than one trial (total pelvic floor versus postanal repair, 44 participants) and no trial showed any difference in primary outcome measures.
Authors' conclusions
The review is striking for the lack of high quality randomised controlled trials on faecal incontinence surgery that have been carried out in the last 10 years. Those trials that have been carried out have focused on sacral neuromodulation and injectable bulking agents, both reported in separate reviews. The continued small number of relevant trials identified together with their small sample sizes and other methodological weaknesses limit the usefulness of this review for guiding practice. It was impossible to identify or refute clinically important differences between the alternative surgical procedures. Larger rigorous trials are still needed. However, it should be recognised that the optimal treatment regime may be a complex combination of various surgical and non‐surgical therapies.
Plain language summary
Surgery for faecal incontinence in adults
Faecal incontinence (the inability to control the release of stool) can be debilitating, and is a common reason for older people to need nursing home care. It can happen for many reasons including malformations of the rectum (lower part of the intestine) or anus, neurological (nerve) diseases, or damage during childbirth or surgery. Treatments include pelvic floor muscle training, electrical stimulation, drugs and surgery. Surgery is used in selected groups of people, particularly (but not exclusively) when the defects in the muscles surrounding the anal canal can be corrected mechanically. The review found that there is still not enough evidence on which to judge whether one type of surgical operation was better or worse than another one, or better than different types of treatment for faecal incontinence. However, many of the techniques originally reviewed are now no longer in general use.
Background
Description of the condition
Faecal incontinence, the inability to control the release of stool (Counihan 2005), can be a debilitating problem with medical, social and economic implications. With regard to surgical intervention, three broad clinical groups of individuals can be recognised as suffering from faecal incontinence related to the anal sphincter. These are individuals with simple structural defects of the anal sphincter (for example damage sustained during childbirth), those with a weak but intact anal sphincter and those with complex disruptions of the anal sphincter (for example congenital anorectal malformations, trauma). The group with weak but intact sphincters includes different aetiologies (diabetes, neurological disorders, spinal trauma, rectal prolapse and primary muscular degeneration) (Kamm 1998; Vaizey 1998).
The true prevalence of faecal incontinence in the community remains uncertain, with considerable variation in published data largely due to variations in case definition, but for anal incontinence (incontinence to flatus and faeces) this is thought to be approximately 2% to 17% (Johanson 1996; Kalantar 2002; Lam 1999; Nakayama 1997; Nelson 1995; Perry 2002; Siproudhis 2006; Whitehead 2009). However defined, the prevalence amongst elderly individuals living in care facilities appears to be greatest. For women over 65 years and living in their own homes the estimated prevalence of faecal incontinence in the UK is 10% to 20% whilst the corresponding figure amongst men is 7% to 10%. The prevalence for both sexes rises to 25% for those living in residential homes and 40% for those living in nursing homes (Turnberg 1995). Amongst older people faecal incontinence remains a major reason for admission to nursing home care (Kamm 1998). Amongst younger women, faecal incontinence may be present after 4% of vaginal deliveries (MacArthur 1997).
In the United States the average annual cost of treating a patient with mixed urinary and faecal incontinence in an outpatient setting was estimated at USD 17,166 (Mellgren 1999). During 1999 the direct costs of pads, appliances and other prescription items throughout hospitals and long term care settings in the UK for incontinence in general was estimated at GBP 82.5 million (Integrated continence service 2000). With the rise in numbers of elderly people in the world, this condition will be an increasing challenge to both healthcare services and home carers.
Description of the intervention
Normal continence of faeces is maintained by a series of neural reflexes (and voluntary activity) acting on specific muscle groups (Sun 1990; Uher 1998). These muscles include the smooth internal and striated external anal sphincters and the pelvic floor muscles (pubococcygeus, ileococcygeus, coccygeus and puborectalis). Control of the neural reflexes (somatic and autonomic) occurs at the level of the rectum (Burnstock 1990) and within centres in the brain stem and frontal lobes (Andrews 1964). However, the precise integration of information between these centres and the interaction between both the somatic and autonomic nervous system remains to be explained (de Groat 1990).
A number of treatment options are currently available for faecal incontinence. These range from conservative measures aimed at symptomatic control (for example dietary manipulation, absorbent pads, anal plugs (including electrically stimulated plugs (Osterberg 2004)), and pharmacotherapy including hormonal manipulation, constipating agents, enemas, laxatives and suppositories) through to interventions aimed at correcting the underlying cause. Interventions attempting to cure the condition include both non‐operative and operative techniques. Non‐operative procedures include biofeedback and pelvic floor muscle training.
Non‐operative procedures do not correct the underlying faulty continence mechanism but instead aim to reduce the symptoms and inconvenience of faecal incontinence by creating an irrigation system (for example percutaneous endoscopic colostomy, Malone antegrade colonic enema) or anal bypass (colostomy or ileostomy). Sacral nerve stimulation, in which electrodes are inserted through the sacral foramina under general anaesthetic for stimulation of the sacral nerves, is being considered in a separate Cochrane review (Mowatt 2007). Bulking agents are also considered in a separate Cochrane review (Maeda 2009).
Operative intervention is usually carried out if appropriate assessment (including clinical history with endoanal ultrasound and physiological evaluation of the neuromuscular units) indicates the presence of a surgically correctable defect. Such surgical procedures usually involve repairing the defect. Other surgical procedures aim to augment the pelvic floor either by bulking of the sphincter, the formation of a neo‐sphincter, or stimulation of the pelvic nerves (Pescatori 2004).
How the intervention might work
The surgical interventions being evaluated are of five types.
I. Interventions designed to correct abnormalities of the pelvic floor
Anterior levatorplasty
A curved perineal incision anterior to the anal orifice is made. The levator ani muscles are identified either side of the pelvis and levatorplasty performed by placing a series of interrupted sutures through each side and tying the sutures when all have been placed. An anterior levatorplasty not only repairs any occult anterior tear, but also increases the length of the anal canal.
Postanal pelvic floor repair
A V‐shaped incision is made posterior to the anal orifice. The tissues are dissected to the internal and external sphincters, which are separated. The puborectalis muscle is separated from the rectum providing direct access to the superior aspect of the pelvic floor muscles. A series of sutures are placed in the two limbs of the pelvic floor including the puborectalis, forming a lattice across the pelvis. The theory behind the postanal repair is restoration of an acute anorectal angle, improving continence by creating a flap valve effect of the puborectalis sling.
Total pelvic floor repair
This combines anterior levatorplasty and postanal repair.
II. Interventions designed to correct abnormalities of the native anal sphincter
Anterior sphincter repair
A circumferential perineal skin or posterior fourchette incision is made and deepened through the ischiorectal fat. The ends of the sphincter muscle are identified and overlapped or apposed by a series of sutures.
Sphincter plication (external sphincter intact)
The external sphincter muscle is dissected free. The muscle is divided or simply overlapped sufficiently to decrease the anal orifice. A series of mattress sutures are then placed to maintain the desired aperture.
III. Interventions designed to create a new anal sphincter (neosphincter) without electrical stimulation
This surgical option is reserved for circumstances where direct repair of the sphincter complex is considered inappropriate.
In one technique, skeletal muscle is imported from an adjacent site and wrapped around the anal canal creating a new sphincter (neosphincter). A number of muscles have been used including the gracilis, gluteus maximus and obturator internus.
Alternatively an artificial sphincter using a hydraulic silastic balloon cuff (for example Acticon or PAS) or a ring of minature magnets may be used (for example Fenix).
Also included in this group are the devices that attempt to tighten or bulk up the anal sphincter (for example Thiersch wires; injectable bulking materials such as silicone, collagen, autologous fat and Teflon paste; and the SECCA procedure thought to work by applying temperature‐controlled radiofrequency energy to cause collagen contraction, healing and remodelling of muscle fibres (Takahashi 2002)).
A recent area of research that currently remains experimental is the use of progenitor cells derived from skeletal muscle myoblasts to replace or repair a damaged sphincter (EUCTR 2010; Michot 2012).
IV. Interventions designed to create a new anal sphincter (neosphincter) with electrical stimulation
This is similar to the above muscle neosphincter procedure except that a nerve stimulator is implanted within the muscle wrap.
V. Interventions designed to create antegrade colonic irrigation
These procedures aim to produce a discrete stoma for the intermittent irrigation of the colon allowing defaecation at a convenient time and reducing incontinent episodes at inappropriate times by creating a clean colon. Techniques include the use of an appendiceal or ileal tube, or a percutaneous endoscopically or radiologically placed tube.
Why it is important to do this review
The focus of this review was to assess surgical techniques in current clinical practice for faecal incontinence. Techniques aimed at the management of faecal incontinence secondary to rectal prolapse were not included and are the subject of a separate Cochrane review by the Incontinence Group (Tou 2008), as are sacral nerve stimulation (Mowatt 2007) and bulking agents (injectables) (Maeda 2009).
Objectives
To assess the performance of surgical techniques for the treatment of faecal incontinence in adults who do not have rectal prolapse. Our aim was firstly to compare surgical techniques with non‐surgical methods and secondly, to compare the various surgical techniques with each other.
Two main comparisons were addressed:
1. surgical techniques versus non‐surgical methods; and
2. one surgical technique with another.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of surgery in the management of faecal incontinence (other than surgery for rectal prolapse or sacral nerve stimulation).
Types of participants
All adults with faecal incontinence as defined by the trialists. Children were excluded as the aetiology of incontinence is more diverse and complex (for example congenital disorders).
Types of interventions
Five types of surgical intervention have been considered.
I. Interventions designed to correct abnormalities of the pelvic floor. II. Interventions designed to correct abnormalities of the native anal sphincter. III. Interventions designed to create a new anal sphincter (neosphincter) without electrical stimulation. IV. Interventions designed to create a new anal sphincter (neosphincter) with electrical stimulation. V. Interventions designed to create antegrade colonic irrigation.
Types of outcome measures
There is a range of perspectives from which the outcome of surgery for faecal incontinence can be viewed and this is reflected in the list of measures reported below. Nevertheless, because of the dangers of multiple statistical testing and data dependent reporting, three measures of poor outcome have been selected as primary measures. These have been presented in tabular form regardless of whether or not data were available. Data describing the other, secondary, outcomes have also been sought but were only tabulated if the data were available.
Primary outcomes
No change or deterioration in incontinence (no improvement)
Failure to achieve full continence
Presence of faecal urgency
Secondary outcomes
Patient symptoms
Patient assessed measures of incontinence (patient assessment of outcome, need to wear absorbent products for incontinence, restriction in activities)
Incontinence score
Clinical end points
Post‐operative complications: bleeding, post‐operative infection, chronic wound pain
Adverse functional events: post‐operative constipation, impaired rectal emptying
Need for additional therapy: drugs, dietary changes, repeat surgery for faecal incontinence including formation of a colostomy
Length of hospital stay
Post‐operative mortality
Physiological measures
Maximum resting anal pressure
Maximum squeeze pressure
Mucosal electrosensitivity
Rectal capacity
Anal canal length
Health status measures
Condition specific health status measures (e.g. FIQL)
Psychological measures (e.g. the Hospital Anxiety and Depression Scale by Zigmond 1983)
General health measures (e.g. the Short Form‐36 (SF‐36) by Ware 1993)
Health economics measures
Other outcomes
Non‐prespecified outcomes judged important when performing the review
Search methods for identification of studies
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Review Group.
Relevant trials were identified from the Incontinence Group Specialised Register of controlled trials, which is described under the Incontinence Group module in The Cochrane Library. The register contains trials identified from MEDLINE, MEDLINE in process, the Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings. The date of the most recent search of the Register for this review was 6 March 2013. The trials in the Incontinence Group Specialised Register are also contained in CENTRAL.
The terms used to search the Incontinence Group Specialised Register are given below: (TOPIC.FAECAL INCONTINENCE*) AND ({DESIGN.CCT*} OR {DESIGN.RCT*}) AND (INTVENT.SURG*) (all searches were of the keyword field of Reference Manager 12, Thomson Reuters).
The Specialised Register of the Cochrane Colorectal Cancer Group was also searched for the previous version of the review. For this update, after contact with the Colorectal Group, the search of CENTRAL was recommended by them as it covers their Register (see below).
For this review the review authors also performed additional specific searches. These included systematic searches of the following electronic databases:
PubMed (1 January 1950 to 6 March 2013) using the following search terms: faecal (or fecal) incontinence (limited to randomised controlled trial);
EMBASE (on Ovid online) (1 January 1998 to 6 March 2013) using the following strategy: fecal incontinence/ OR (faecal or fecal and incontinent$).tw. These terms were combined using the Boolean operator 'OR';
CENTRAL (2013, Issue 1).
Searching other resources
The review authors also specifically searched all the reference lists of relevant articles, and handsearched the following journals and conference proceedings:
British Journal of Surgery (from January 1995 to March 2013);
Diseases of the Colon and Rectum (from January 1995 to March 2013);
Colorectal Diseases (from January 2000 to March 2013); and
conference proceedings of the Association of Coloproctology of Great Britain and Ireland annual meetings (from 1999 to 2012).
Data collection and analysis
Selection of studies
Three review authors (SRB, HW, RLN) examined all the citations and abstracts derived from the electronic searches. Reports of potentially relevant trials were retrieved in full. The three review authors independently applied the selection criteria to the trials reports. Review authors were not blind to the names of authors, institutions or journals. Any disagreements were resolved by discussion.
Data extraction and management
Data extraction from the included trials was undertaken independently by the three review authors (RLN, HW, SRB). Data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) Any difference of opinion was resolved by discussion between the review authors. Some data required extraction from figures in the publications. An attempt was made to get missing information from the trial's authors and it was obtained for one trial (Leroi 2005). All other data were published data only.
Assessment of risk of bias in included studies
The methodological quality of the identified trials was assessed independently by the three review authors, taking into account the quality of random allocation concealment and the description of dropouts and withdrawals as well as blinding of the patients and carers to the intervention. Any disagreements were resolved by discussion. Studies were excluded if they were not randomised or quasi‐randomised controlled trials in adults. The excluded studies and the reasons for their exclusion are summarised in the table 'Characteristics of excluded studies'.
Data synthesis
Data were analysed using the RevMan statistical programme in Review Manager.
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for dichotomous outcomes using the Mantel‐Haenszel method and a fixed‐effect model. Continuous variables were analysed using fixed‐effect model meta‐analyses of (weighted) mean differences (MDs). Continuous variables were processed using the means and standard deviations. Where the results from trials had been reported in terms of the mean and standard error of the mean (SEM), then the standard deviation (SD) was calculated using the relationship defined by the equation: SD = SEM x sample size 1/2.
Subgroup analysis and investigation of heterogeneity
These analyses were not used as the number of trials for each type of intervention was never more than two.
Results
Description of studies
Results of the search
A total of 230 records were identified through the literature search and the titles and abstracts were assessed. The full text articles of 30 potentially relevant studies were obtained. Nine randomised controlled trials (RCTs) were identified and included in this review. The flow of literature through the assessment process is shown in Figure 1.
1.
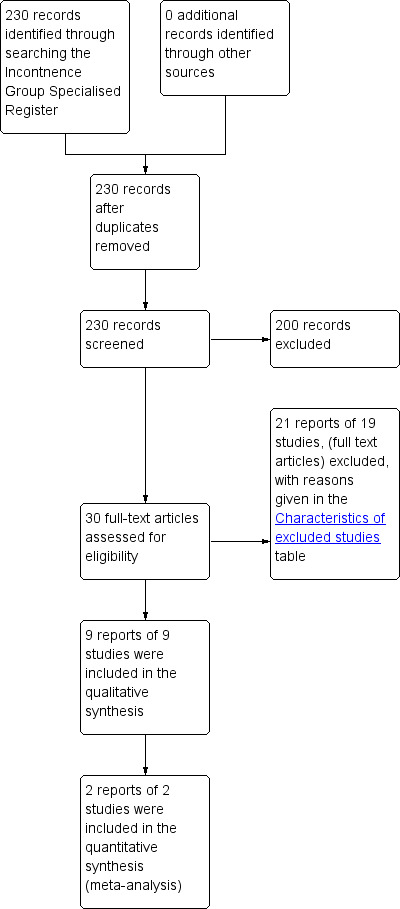
PRISMA study flow diagram.
Included studies
Two trials compared surgical with non‐surgical interventions and the remaining seven trials compared different surgical interventions or the same surgical intervention with or without an adjunct treatment. There were a total of 264 patients included in the nine trials of which only 10 were male. Six trials limited participants to those with idiopathic incontinence (no obvious structural sphincter defect) as the main cause of their symptoms (Deen 1993; Deen 1995; O'Brien 2004; Osterberg 2004; van Tets 1998; Yoshioka 1999) while three trials included only those who had localised external sphincter defects (Davis 2004; Hasegawa 2000; Tjandra 2003).
Each of the nine trials was performed at a single surgical centre, five in the United Kingdom (Davis 2004; Deen 1993; Deen 1995; Hasegawa 2000; Yoshioka 1999), two in Australia (O'Brien 2004; Tjandra 2003), one in Sweden (Osterberg 2004) and one in the Netherlands (van Tets 1998). All had small sample sizes (range 20 to 59). Post‐operative follow‐up periods extended up to 47 months (Hasegawa 2000). Where data were recorded from various follow‐up periods, the data were analysed from the close of the study only. Further details of these nine trials are presented in the table 'Characteristics of included studies'.
Excluded studies
Although previously included, injection of bulking agents has now been removed from this review as this intervention is covered in another review (Maeda 2009). The excluded studies on bulking agents consisted of the original four trials covered in the last update (Maeda 2007; Siphrouis 2004; Tjandra 2004; Tjandra 2009) with two additional trials (Dehli 2013; Graf 2011).
Eight trials were identified that compared overlap external sphincter repair with end to end repair (Farrell 2010; Farrell 2012; Fernando 2006a; Fitzpatrick 2000; Garcia V 2005; Nordenstam 2008; Rygh 2010; Williams 2004). These trials were excluded because they examined repair in the acute obstetric injury scenario. As the study participants had not necessarily reported faecal incontinence symptoms before the repair these studies did not strictly meet the criteria for inclusion. They are also covered in another Cochrane review (Fernando 2006b).
Four trials (Leroi 2005; Michelsen 2008; Tjandra 2008; Vaizey 1999) addressed sacral nerve stimulation, which is also covered in another Cochrane review (Mowatt 2007).
The last study did not provide enough data to determine whether it should be included (Loder 1993). Further details of all of these trials are presented in the Characteristics of excluded studies table.
Risk of bias in included studies
All of the trials had methodological drawbacks, which may have resulted in significant selection, performance, attrition and detection bias.
Allocation
It was unclear in three studies as to how the participants were randomised (Hasegawa 2000; Tjandra 2003; Yoshioka 1999). Randomisation was reported in four studies to have been performed by 'random numbers' (Davis 2004; Deen 1993; Deen 1995; O'Brien 2004) and in one each by 'random blocks' (Osterberg 2004) or 'random lots' (van Tets 1998). Even for those trials that mentioned the randomisation process, only one trial (Osterberg 2004) mentioned allocation concealment.
Blinding
Most trials were open to performance and detection bias because of lack of blinding of the participants and investigators. In many of the trials, particularly those comparing surgical with non‐surgical treatment, blinding was impossible. In one trial of sphincter repair, the participants were blinded to the intervention (Tjandra 2003).
Incomplete outcome data
Four studies did not mention intention to treat (Davis 2004; Maeda 2007; O'Brien 2004; Osterberg 2004). With regard to attrition bias only three trials (Davis 2004; Hasegawa 2000; Osterberg 2004) provided information regarding withdrawals.
Other potential sources of bias
Three trials (Deen 1995; O'Brien 2004; Osterberg 2004) provided details regarding statistical power, minimum clinical differences sought and sample size calculation. In one of these trials (Deen 1995) there was data dependent stopping of the trial after recruiting 30 patients due to a measured reduction in the maximum resting anal pressure following total pelvic floor repair with internal sphincter plication. The value of data from some of the trials was further limited by the published data being presented in a form not directly usable by the review authors, such as merely expressing a statistical comparison in terms of a P value, or the use of medians and ranges rather than means plus or minus the standard deviation (Figure 2; Figure 3).
2.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
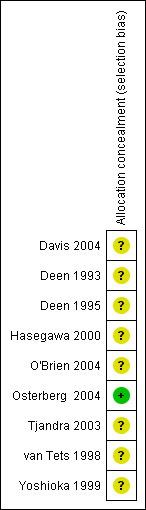
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
A baseline evaluation was not reported in two studies (Tjandra 2003; Yoshioka 1999). No differences in baseline demographics or symptomatology were reported between groups in all studies except one (Deen 1995) where patients undergoing total pelvic floor repair were reported to have suffered incontinence symptoms for longer. The effectiveness of the surgical intervention in each trial was assessed using a variety of outcome measures.
Effects of interventions
1. Surgical versus non‐surgical intervention
We identified two trials which compared surgical with non‐surgical treatment of individuals with faecal incontinence.
I. Interventions designed to correct abnormalities of the pelvic floor
Levatorplasty versus anal plug electrostimulation of the pelvic floor
One primary outcome (number of patients having no change or deterioration in continence) was reported. Five of 31 patients after anterior levatorplasty and nine of 28 patients after anal plug electrostimulation did not improve (OR 0.41, 95% CI 0.12 to 1.41, Analysis 1.1).
1.1. Analysis.
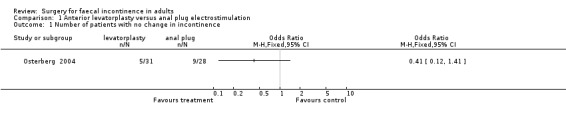
Comparison 1 Anterior levatorplasty versus anal plug electrostimulation, Outcome 1 Number of patients with no change in incontinence.
Of the eight secondary outcomes, data suitable for analysis were unobtainable for four (incontinence score and physiology measures). For two outcomes (use of pads and complications) there were no statistically significant differences between the two groups. However, patient assessments of physical and social handicap (yes or no question) were significantly in favour of the levatorplasty (OR 0.19, 95% CI 0.06 to 0.59, Analysis 1.6) and OR 0.05, 95% CI 0.01 to 0.29, Analysis 1.7), respectively.
1.6. Analysis.
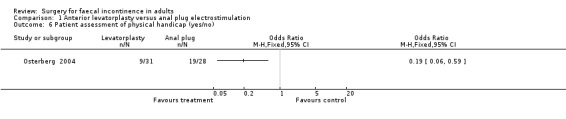
Comparison 1 Anterior levatorplasty versus anal plug electrostimulation, Outcome 6 Patient assessment of physical handicap (yes/no).
1.7. Analysis.
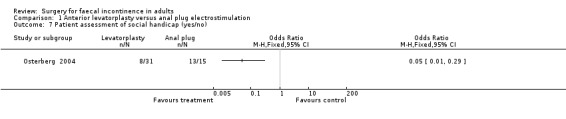
Comparison 1 Anterior levatorplasty versus anal plug electrostimulation, Outcome 7 Patient assessment of social handicap (yes/no).
II. Interventions designed to correct abnormalities of the native anal sphincter
There were no studies comparing surgical correction of sphincter abnormality with conservative treatment.
III. Interventions designed to create a new anal sphincter (neosphincter) without electrical stimulation
Artificial bowel sphincter versus supportive care
Although no primary outcomes were given directly it was possible to infer from the text and tables that all six patients who had a successful implant showed some improvement in continence while only one of seven patients in the control group improved (OR 0.02, 95% CI 0.0 to 0.52, Analysis 2.1).
2.1. Analysis.
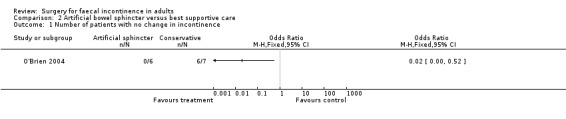
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 1 Number of patients with no change in incontinence.
Secondary outcome measures included an incontinence score, incontinence specific quality of life score (Aging Males' Symptoms (AMS) QoL) and SF‐36 quality of life score (mental and physical components). The incontinence score, specific quality of life score and generic mental QoL score statistically favoured the surgical intervention (MD 9.5, 95% CI 4.8 to 14.2, Analysis 2.4; MD 28, 95% CI 5.7 to 50.3, Analysis 2.5; and MD 7.6, 95% CI 2.70 to 12.50, Analysis 2.7, respectively). The physical component of the generic quality of life score (SF‐36) and the depression score were not statistically different. Complications occurred in three of the seven in the surgical group, one person requiring abandonment of the device and stoma formation (OR 11.67, 95% CI 0.48 to 282.04, Analysis 2.9).
2.4. Analysis.
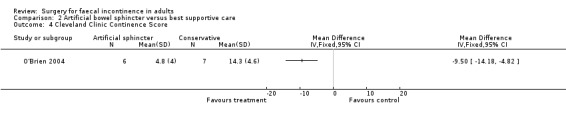
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 4 Cleveland Clinic Continence Score.
2.5. Analysis.
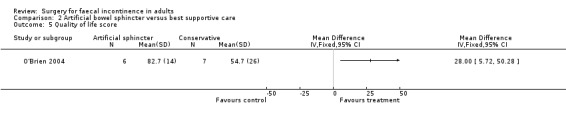
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 5 Quality of life score.
2.7. Analysis.
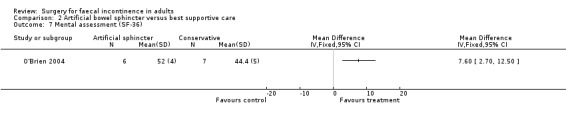
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 7 Mental assessment (SF‐36).
2.9. Analysis.
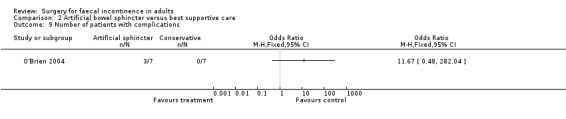
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 9 Number of patients with complications.
IV. Interventions designed to create a new anal sphincter (neosphincter) with electrical stimulation
There were no studies comparing a stimulated neosphincter with conservative treatment.
V. Interventions designed to create an anterograde colonic irrigation system
There were no studies comparing anterograde colonic irrigation techniques with conservative management.
2. Surgical versus surgical interventions
We identified seven trials which compared different surgical approaches to the treatment of individuals with faecal incontinence. All the trials were small, and almost all the outcomes were only addressed by single trials.
I. Interventions designed to correct abnormalities of the pelvic floor
a) Anterior levatorplasty versus postanal repair
One primary outcome measure, number of patients failing to achieve full continence, was reported. Eight of 12 patients after anterior levatorplasty and seven of 12 after postanal repair failed to achieve full continence (OR 1.41, 95% CI 0.28 to 7.09, Analysis 3.2).
3.2. Analysis.
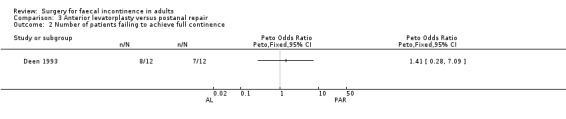
Comparison 3 Anterior levatorplasty versus postanal repair, Outcome 2 Number of patients failing to achieve full continence.
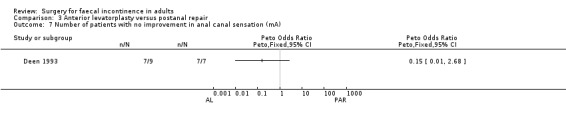
Of the five secondary outcome measures reported (adverse functional effects, maximum resting anal and squeeze pressures, changes in anal canal sensation and functional anal canal length) only one (dyspareunia) was found to be statistically significantly different between the groups, in favour of postanal repair (five out of 12 versus zero out of 12; OR 11.26, 95% CI 1.64 to 77.47, Analysis 3.4).
3.4. Analysis.
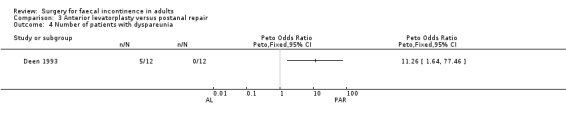
Comparison 3 Anterior levatorplasty versus postanal repair, Outcome 4 Number of patients with dyspareunia.
b) Total pelvic floor repair versus anterior levatorplasty
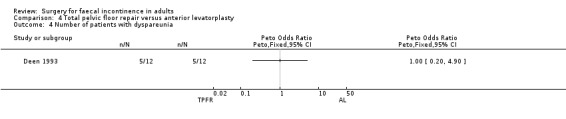
Only one primary outcome measure, number of patients failing to achieve full continence, was reported. Four of 12 participants after total pelvic floor repair compared to eight of 12 participants after anterior levatorplasty failed to achieve full continence (OR 0.28, 95% CI 0.06 to 1.33, Analysis 4.2).
4.2. Analysis.
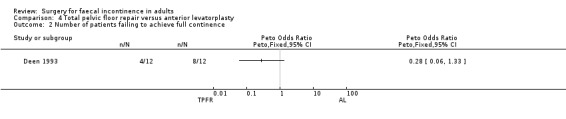
Comparison 4 Total pelvic floor repair versus anterior levatorplasty, Outcome 2 Number of patients failing to achieve full continence.
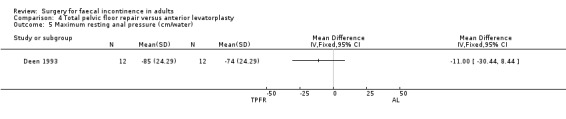
Of the secondary outcome measures, five patients experienced dyspareunia after each intervention. Maximum resting anal and squeeze pressures and anal canal length remained unchanged. Ten of 10 patients after total pelvic floor repair and seven of nine patients after anterior levatorplasty failed to show an improvement in anal canal sensation after surgery.
c) Total pelvic floor repair versus postanal repair
Two primary outcome measures were reported: number of patients with no change in incontinence (van Tets 1998), and number of patients failing to achieve full continence (Deen 1993; van Tets 1998). In one trial (van Tets 1998) six of 11 patients after total pelvic floor repair and six of nine patients after postanal repair showed no change in incontinence (OR 0.62, 95% CI 0.11 to 3.57, Analysis 5.1). In the combined comparisons (Deen 1993; van Tets 1998) data from only one trial (Deen 1993) were available, with four of 12 patients after total pelvic floor repair and seven of 12 patients after postanal repair failing to achieve full continence (OR 0.38, 95% CI 0.08 to 1.84, Analysis 5.2); whereas this applied to all 20 patients in the van Tets trial (van Tets 1998).
5.1. Analysis.
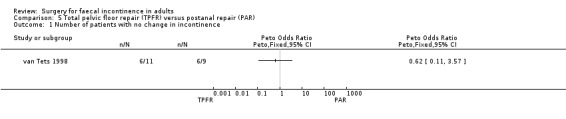
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 1 Number of patients with no change in incontinence.
5.2. Analysis.
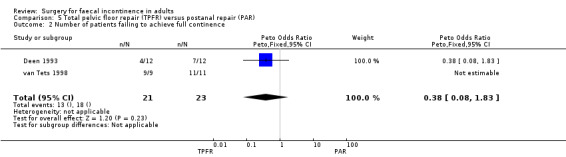
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 2 Number of patients failing to achieve full continence.
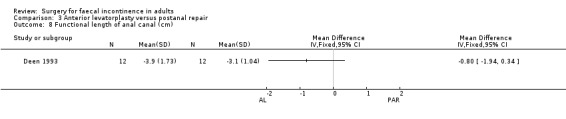
Of the secondary outcome measures, significantly fewer adverse functional events occurred after postanal repair (zero of 12) compared to total pelvic floor repair (five of 12) (OR 11.26, 95% CI 1.64 to 77.47, Analysis 5.4). The functional length of the anal canal appeared to be improved after total pelvic floor repair (4.70 cm) compared with postanal repair (3.10 cm; weighted mean difference (WMD) 1.6 cm, 95% CI 0.28 to 2.92, Analysis 5.8). For the remaining physiological measures (maximum squeeze and resting anal pressures) no differences between the groups were observed.
5.4. Analysis.
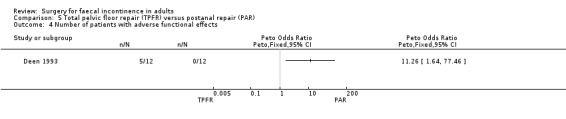
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 4 Number of patients with adverse functional effects.
5.8. Analysis.
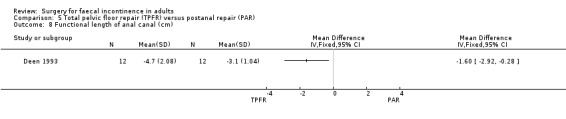
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 8 Functional length of anal canal (cm).
II. Interventions designed to correct abnormalities of the native anal sphincter
a) Overlap sphincter repair versus direct end to end repair
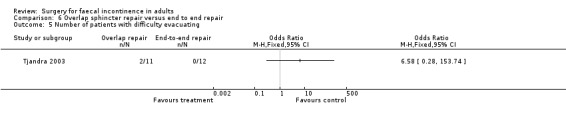
The only study to look at overlap versus end to end repair in the non‐acute (secondary repair not primary at time of rupture) setting had one primary end point. Three of 11 patients undergoing overlap repair showed no improvement in continence compared with three of the 12 patients who underwent direct repair (OR 1.13, 95% CI 0.17 to 7.24, Analysis 6.1).
6.1. Analysis.
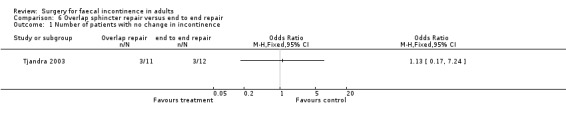
Comparison 6 Overlap sphincter repair versus end to end repair, Outcome 1 Number of patients with no change in incontinence.
Of the secondary outcomes there were again no statistically significant differences for the continence and physiology scores, the use of imodium, adverse function or complications.
b) Overlap sphincter repair versus overlap repair with defunctioning of the bowel
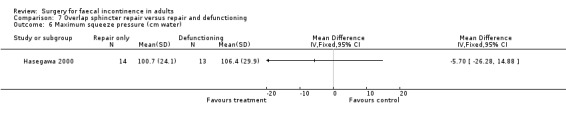
There were no primary outcome measures. Of the secondary outcome measures there were no statistical differences in continence score and physiological parameters. With regard to complications, seven of 13 patients suffered stoma‐related problems but the incidence of sphincter repair‐related problems was similar (OR 0.44, 95% CI 0.08 to 2.38, Analysis 7.7). Not surprisingly the overall hospital stay was significantly greater for the stoma group due to the readmissions for stoma closure (WMD 7.8, 95% CI 4.74 to 10.86, Analysis 7.8).
7.7. Analysis.
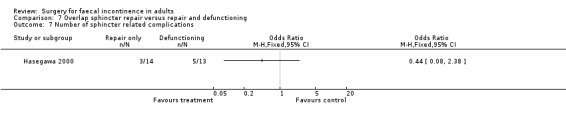
Comparison 7 Overlap sphincter repair versus repair and defunctioning, Outcome 7 Number of sphincter related complications.
7.8. Analysis.
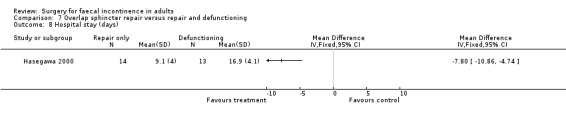
Comparison 7 Overlap sphincter repair versus repair and defunctioning, Outcome 8 Hospital stay (days).
c) Overlap sphincter repair and levatorplasty versus the same repair with biofeedback
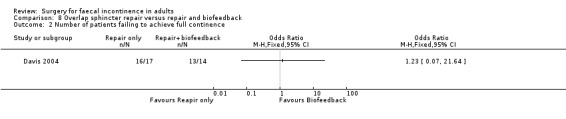
Two primary outcomes were reported. Only one patient from each group achieved complete continence. However, patient self‐evaluation suggested that only one patient of 14 from the repair and biofeedback group did not improve compared with six out of 17 from the repair only group (Analysis 8.1).
8.1. Analysis.
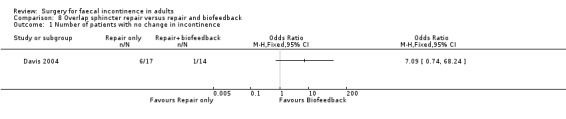
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 1 Number of patients with no change in incontinence.
Of the secondary outcomes there was no difference seen with continence, patient satisfaction or physiological scores. However, there were statistically significant differences in favour of repair with biofeedback for some of the domains of the quality of life scores: higher lifestyle score (MD 0.76, 95% CI 0.31 to 1.21, Analysis 8.6); less depression (MD 0.75, 95% CI 0.16 to 1.34, Analysis 8.8); and less embarrassment (MD 0.83, 95% CI 0.12 to 1.54, Analysis 8.9).
8.6. Analysis.
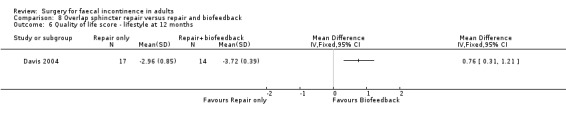
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 6 Quality of life score ‐ lifestyle at 12 months.
8.8. Analysis.
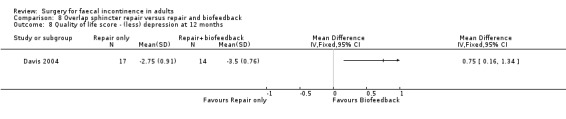
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 8 Quality of life score ‐ (less) depression at 12 months.
8.9. Analysis.
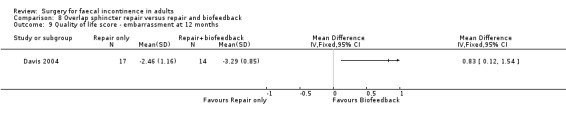
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 9 Quality of life score ‐ embarrassment at 12 months.
d) Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair alone
No data were available for analysis for any of the primary outcome measures.
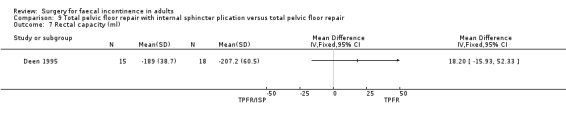
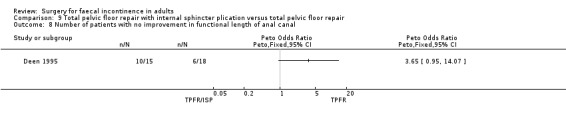
A limited number of secondary outcome measures were reported. Maximum resting anal pressure showed a statistically significant difference in favour of the total pelvic floor repair alone group after surgery (MD 23.69, 95% CI 6.37 to 41.0, Analysis 9.5). However, this was not accompanied by a significant difference in the incontinence scores between the two groups (Analysis 9.4). Similarly, the remaining physiological outcome measures (mucosal electrosensitivity, rectal capacity and functional anal canal length) showed no significant difference between the two treatment groups. One of 15 patients after total pelvic floor repair and internal sphincter plication compared with two of 18 patients after total pelvic floor repair alone suffered post‐operative complications (Analysis 9.9).
9.5. Analysis.
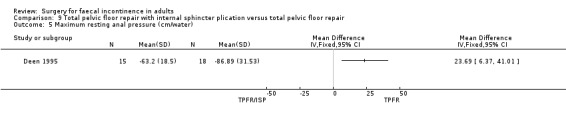
Comparison 9 Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair, Outcome 5 Maximum resting anal pressure (cm/water).
9.4. Analysis.
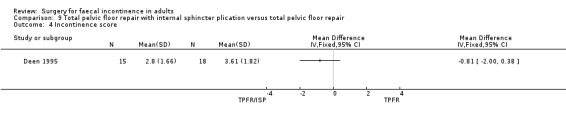
Comparison 9 Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair, Outcome 4 Incontinence score.
9.9. Analysis.
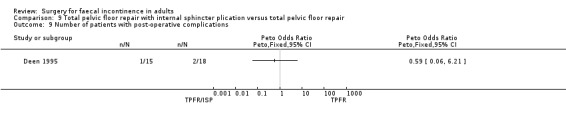
Comparison 9 Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair, Outcome 9 Number of patients with post‐operative complications.
III. Interventions designed to create a new anal sphincter (neosphincter) without electrical stimulation
Comparison of gluteus maximus transposition (GMT), with total pelvic floor repair (TPFR)
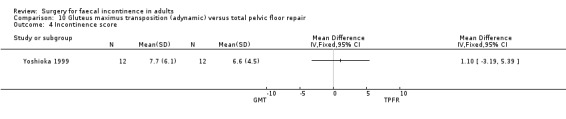
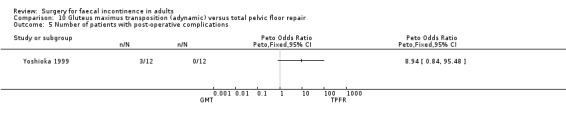
Data were available for two of the primary outcome measures: number of patients failing to achieve full continence (Analysis 10.2), and number of patients with no improvement in faecal urgency (Analysis 10.3). There were no significant differences seen between the two groups.
10.2. Analysis.
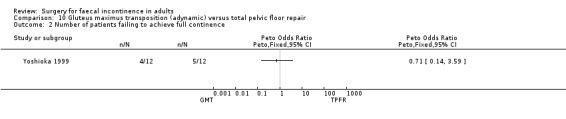
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 2 Number of patients failing to achieve full continence.
10.3. Analysis.
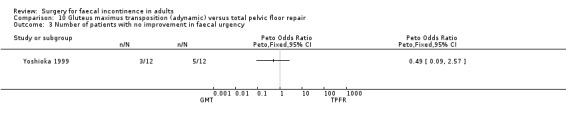
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 3 Number of patients with no improvement in faecal urgency.
No statistically significant differences were identified between the groups for any of the secondary outcome measures (continence score, adverse effects, mean resting anal pressure, mucosal electrosensitivity, maximum squeeze pressure and length of high pressure zone).
IV. Interventions designed to create a new anal sphincter (neosphincter) with electrical stimulation
There were no studies comparing formation of a new anal sphincter with electrical stimulation.
V. Interventions designed to create an antegrade colonic irrigation system
There were no studies comparing anterograde colonic irrigation techniques with other surgical interventions.
Discussion
Summary of main results
Surgical intervention has been advocated for individuals with faecal incontinence in appropriate cases since the operation of postanal repair was first popularised (Parks 1975). In the intervening period, improvements to surgical care and the development of new surgical options have continued to occur. However, there still appears to be no consensus on what defines optimum surgical management. Although there continues to be a few randomised controlled trials produced since the original Cochrane review, and there are some trials comparing surgery with non‐surgical procedures, the results of the updated review still fail to provide robust evidence in favour of any of the alternative interventions. It should also be noted that most of the included studies are on outdated and seldom used procedures. There is very little evidence on the most commonly used procedures. However, there is weak evidence in favour of using biofeedback to supplement an operation from one small trial (Davis 2004). The use of biofeedback to improve surgical outcomes is the topic of an ongoing clinical trial from Iran (Ghahramani 2013). Another area of ongoing research for commonly performed procedures is the use of a biological implant to reinforce sphincter repair (NCT01044589 2010).
Since the last update, bulking agent injection and sacral neuromodulation are by far the main areas in this field where there is an update in the literature. Both are reported in separate reviews and therefore no longer discussed here. Certainly with regard to neuromodulation, this remains the prime area for technical advances as well as becoming the favoured surgical approach for most patients with faecal incontinence. This probably relates to the minimally invasive nature of the therapy. Ongoing trials include a comparison between bulking agents and neuromodulation for obstetric trauma (Norderval 2012) and an assessment of tibial neuromodulation as an alternative to direct sacral nerve stimulation (Confident trial ISRCTN88559475). However, there are some other techniques for future research including adaptations of the artificial sphincter (the magnetic sphincter (Wong 2011)) and the use of skeletal muscle cell progenitors to repair or augment sphincter function (EUCTR 2010; Michot 2012).
Overall completeness and applicability of evidence
Data reported by the trials appear to be predominantly limited to secondary outcome measures, particularly incontinence and quality of life scores and physiological parameters. Potentially relevant primary outcome measures such as the proportion of patients either failing to achieve full continence or experiencing no change or deterioration in incontinence after surgery were not reported consistently. Incontinence scores and quality of life scores were also not consistently used. Variations make comparisons difficult between studies. However, there has been a move to standardise such scores and to create validated, specific faecal incontinence assessment tools (Rockwood 2000). This may allow more meaningful comparison.
Physiological tests, in particular, appear to be routinely used as proxy measures of immediate effectiveness when comparing outcomes from different intervention groups. Whilst these measures may provide helpful diagnostic information their use as surrogate measures of clinical effectiveness is of concern. These measurements have been reported to be non‐specific with regards to pathology, their values may overlap with those obtained from normal controls, and their results can show wide test‐retest variations (Chen 1998; Felt‐Bersma 1997; Keating 1997; Parellada 1998; Pfeifer 1997; Ryhammer 1997).
The use of endoanal ultrasound examination of patients with faecal incontinence, on which to include or exclude the presence of sphincter defects and consequently allow inclusion into some of the trials, must be interpreted cautiously. Endoanal ultrasound has limitations and identifying anterior defects is particularly difficult. These inaccuracies may result in detection bias.
The morbidity from some interventions was significant and needs to be taken into account when considering the effectiveness of a treatment option. In one study comparing an artifical bowel sphincter with supportive treatment nearly 50% of participants suffered peri‐operative events that required additional procedures or delayed hospital discharge (O'Brien 2004). In another study investigating the need for stoma formation after anterior sphincter repair, significant complications were seen related to the stoma with no apparent difference in the continence outcomes when compared to those without a stoma (Hasegawa 2000).
Quality of the evidence
Methodological weaknesses in many of the trials compromise the value of their results. Unfortunately a continued lack of comparisons between similar interventions allows only limited meta‐analysis. This is an inherent problem with surgery for faecal incontinence where the options for treatment are numerous and the cause of the continence dysfunction in the participants is so varied. In some instances (notably O'Brien 2004 comparing an artificial sphincter with best supportive care) the statistics significantly favoured the treatment option but the numbers were small and analysis was only possible for the one study. There was one instance where there were data from more than one trial. In this comparison (total pelvic floor versus postanal repair), all the participants in both arms of one of the two trials had poor outcomes.
A potential drawback of studies on incontinence is the timing of assessment of outcomes. For many interventions there is deterioration with time. Sphincter repair is the classic example with many long term studies now showing deterioration, with the initial satisfaction rate of 80% at 18 months falling to below 50% after a median of 77 months (Malouf 2000). Outcome data for each included study were taken from the last post‐operative assessment and in only one study (O'Brien 2004) was this less than 12 months after the intervention.
In many of the included trials, randomisation of patients to treatment groups may have been performed using methods that were susceptible to selection bias. Adequate concealment of allocation during treatment and outcome assessment was not evident in any trial; both can potentially bias the results (Byar 1976). The trials described in this review included small numbers of patients and estimates of treatment differences were very imprecise. The follow‐up period was generally inadequate and needs to be considerably longer for a proper evaluation of long term treatment success. For instance, non‐randomised studies have reported that success rates after postanal repair dramatically decline over time and are approximately 26% at six years follow‐up (Rainey 1990; Setti Carraro 1994). The same is true for overlap sphincter repair (Guttierez 2004; Halverson 2002; Malouf 2000).
Authors' conclusions
Implications for practice.
Although there are now some studies comparing surgical intervention with conservative treatment, the interventions are diverse, the outcomes remain varied, and the participant numbers are too small to provide meaningful evidence for practice. With the limited numbers of participants and the single study evidence in mind, there may be some very guarded implications for practice. 1. Routinely defunctioning the rectum by creating a stoma after sphincter repair in uncomplicated cases does not improve the outcome of the repair but does increase morbidity and hospital stay. 2. An artificial bowel sphincter may be better than conservative treatment but has significant morbidity. 3. It does not matter whether a sphincter repair is overlapped or directly apposed, the outcome is the same.
4. It may be that some of the outcomes of surgery can be improved by the use of supplementary biofeedback.
Implications for research.
There is little evidence to either support or refute the efficacy of surgical interventions for faecal incontinence. Uncertainty remains on whether any surgical intervention does more good than non‐surgical treatment. Further multicentre randomised controlled trials with sufficient power are required to evaluate the continuing use of the present surgical interventions for faecal incontinence related to both structural and non‐structural sphincter damage. In addition, the role of physical therapies (pelvic floor muscle training and biofeedback) either as alternatives to surgical intervention or as adjuncts is required to be formally and more frequently investigated as a treatment option.
With careful planning many of the methodological criticisms of present trials (selection bias, blinding of outcome assessors) and problems with clinical trials in general (patient preference, recruitment difficulties, and differences in experience between centres) can be overcome by well‐designed pragmatic randomised controlled trials. Individual trials must have adequate power in order to stand a good chance of detecting clinically significant differences if they exist. Only after such evaluation can patients be offered the optimum evidence based treatment for their condition and ineffective procedures identified. Researchers should carefully consider both patient and surgeon orientated outcome measures, with both inclusion and exclusion criteria which will aid generalisability of the results.
However, the problems with studies on faecal incontinence are not answered by improvements in the methodology alone. The issues are much more complex. For instance, a simple comparison between overlap sphincter repair and other treatments needs to take into account early success as anal sphincter repair decays rapidly (Guttierez 2004; Halverson 2002; Malouf 2000). Why does this occur? How long after the intervention should assessment be carried out? It certainly should be more than a year. Another example of the complexity of any therapeutic comparison is the fact that in practice treatment regimes often involve a combination of surgical and non‐surgical regimes. Studies must consider which combinations work best and in what order.
With the multitude of both surgical and non‐surgical options available for faecal incontinence a robust method of diagnosis and assessment is needed to choose the optimal therapy and this merits further development and evaluation. Most surgeons would agree that endoanal ultrasound is essential to diagnose mechanical damage to the sphincter muscle. However, the question still remains whether anal sphincter surgery is effective for incontinence if no anal sphincter defect is seen on ultrasound.
There are some promising trials that are ongoing and include techniques other than bulking agents and neuromodulation (see discussion). The results of these trials will hopefully be included in the next update.
What's new
| Date | Event | Description |
|---|---|---|
| 17 May 2013 | New citation required but conclusions have not changed | A literature search has been carried out and the text has been updated. No new trials have been added but because there is now a separate review on injectable bulking agents, this section has been removed from the review with references to this review and to the review on sacral nerve stimulation added. |
| 17 May 2013 | New search has been performed | A literature search has been carried out and the text has been updated. No new trials have been added but because there is now a separate review on injectable bulking agents, this section has been removed from the review with references to this review and to the review on sacral nerve stimulation added. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 2 June 2010 | New citation required and conclusions have changed | New studies on injectables added |
| 18 July 2009 | New search has been performed | New literature search and additional relevant papers and reports added |
| 19 June 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
This is the third update of the original Cochrane review written by Bachoo P, Brazzelli M, Grant A. Thanks to the Cochrane Incontinence Group for their help and input into the preparation of the update.
Data and analyses
Comparison 1. Anterior levatorplasty versus anal plug electrostimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with no improvement in faecal urgency | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Number of patients using pads | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Patient assessment of physical handicap (yes/no) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Patient assessment of social handicap (yes/no) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.4. Analysis.
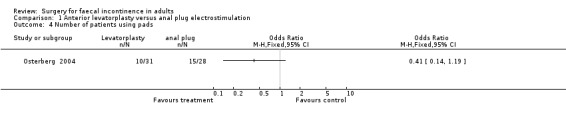
Comparison 1 Anterior levatorplasty versus anal plug electrostimulation, Outcome 4 Number of patients using pads.
1.5. Analysis.
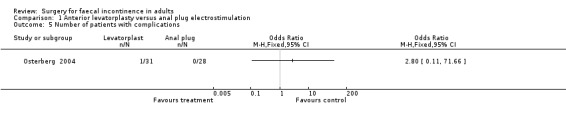
Comparison 1 Anterior levatorplasty versus anal plug electrostimulation, Outcome 5 Number of patients with complications.
Comparison 2. Artificial bowel sphincter versus best supportive care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with no improvement in faecal urgency | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Cleveland Clinic Continence Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of life score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Physical assessment (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Mental assessment (SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Beck depression score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Number of patients with complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.6. Analysis.
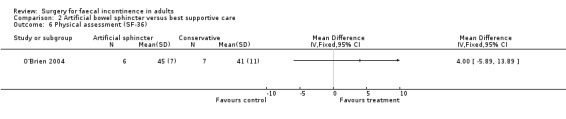
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 6 Physical assessment (SF‐36).
2.8. Analysis.
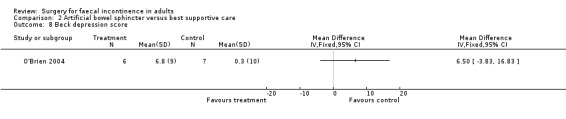
Comparison 2 Artificial bowel sphincter versus best supportive care, Outcome 8 Beck depression score.
Comparison 3. Anterior levatorplasty versus postanal repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with no improvement in faecal urgency | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of patients with dyspareunia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Maximum resting anal pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Maximum squeeze pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of patients with no improvement in anal canal sensation (mA) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8 Functional length of anal canal (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.5. Analysis.
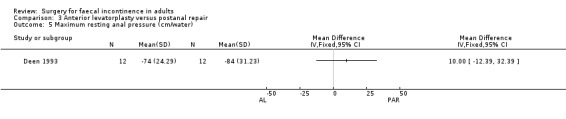
Comparison 3 Anterior levatorplasty versus postanal repair, Outcome 5 Maximum resting anal pressure (cm/water).
3.6. Analysis.
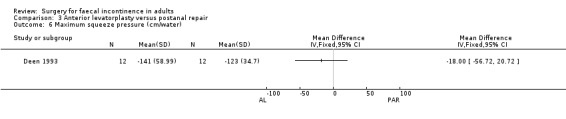
Comparison 3 Anterior levatorplasty versus postanal repair, Outcome 6 Maximum squeeze pressure (cm/water).
3.7. Analysis.
Comparison 3 Anterior levatorplasty versus postanal repair, Outcome 7 Number of patients with no improvement in anal canal sensation (mA).
3.8. Analysis.
Comparison 3 Anterior levatorplasty versus postanal repair, Outcome 8 Functional length of anal canal (cm).
Comparison 4. Total pelvic floor repair versus anterior levatorplasty.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with no improvement in faecal urgency | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Number of patients with dyspareunia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Maximum resting anal pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Maximum squeeze pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of patients with no improvement in anal canal sensation (mA) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8 Functional length of anal canal (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.4. Analysis.
Comparison 4 Total pelvic floor repair versus anterior levatorplasty, Outcome 4 Number of patients with dyspareunia.
4.5. Analysis.
Comparison 4 Total pelvic floor repair versus anterior levatorplasty, Outcome 5 Maximum resting anal pressure (cm/water).
4.6. Analysis.
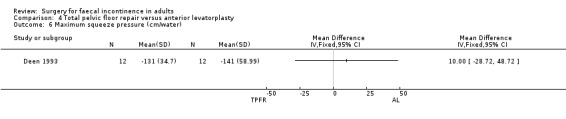
Comparison 4 Total pelvic floor repair versus anterior levatorplasty, Outcome 6 Maximum squeeze pressure (cm/water).
4.7. Analysis.
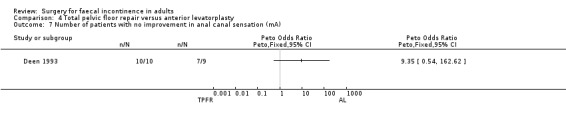
Comparison 4 Total pelvic floor repair versus anterior levatorplasty, Outcome 7 Number of patients with no improvement in anal canal sensation (mA).
4.8. Analysis.
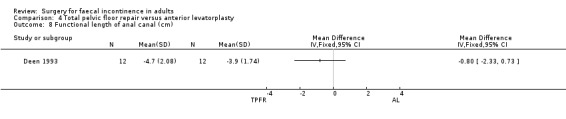
Comparison 4 Total pelvic floor repair versus anterior levatorplasty, Outcome 8 Functional length of anal canal (cm).
Comparison 5. Total pelvic floor repair (TPFR) versus postanal repair (PAR).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 2 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.83] |
| 3 Number of patients with no improvement in faecal urgency | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of patients with adverse functional effects | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Maximum resting anal pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Maximum squeeze pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of patients with no improvement in anal canal sensation (mA) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8 Functional length of anal canal (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Number of patients with a decrease in maximum squeeze pressure | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
5.5. Analysis.
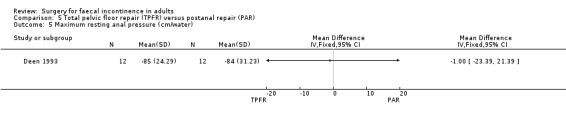
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 5 Maximum resting anal pressure (cm/water).
5.6. Analysis.
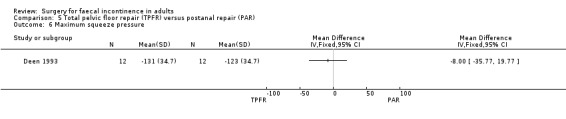
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 6 Maximum squeeze pressure.
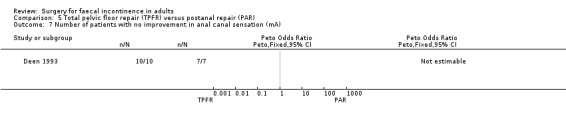
5.7. Analysis.
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 7 Number of patients with no improvement in anal canal sensation (mA).
5.9. Analysis.
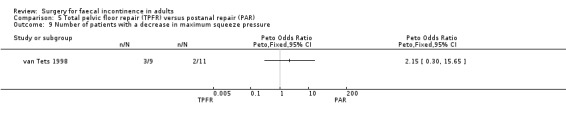
Comparison 5 Total pelvic floor repair (TPFR) versus postanal repair (PAR), Outcome 9 Number of patients with a decrease in maximum squeeze pressure.
Comparison 6. Overlap sphincter repair versus end to end repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number of patients with no improvement in faecal urgency | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of patients having to use imodium | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with difficulty evacuating | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.4. Analysis.
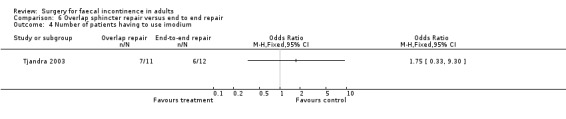
Comparison 6 Overlap sphincter repair versus end to end repair, Outcome 4 Number of patients having to use imodium.
6.5. Analysis.
Comparison 6 Overlap sphincter repair versus end to end repair, Outcome 5 Number of patients with difficulty evacuating.
6.6. Analysis.
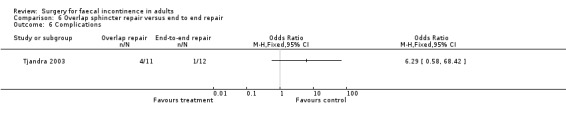
Comparison 6 Overlap sphincter repair versus end to end repair, Outcome 6 Complications.
Comparison 7. Overlap sphincter repair versus repair and defunctioning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of patients failing to achieve full continence | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number of patients with no improvement in faecal urgency | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cleveland Clinic Continence Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Maximum resting pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Maximum squeeze pressure (cm water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Number of sphincter related complications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
7.4. Analysis.
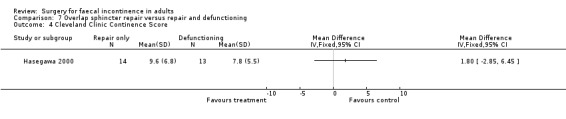
Comparison 7 Overlap sphincter repair versus repair and defunctioning, Outcome 4 Cleveland Clinic Continence Score.
7.5. Analysis.
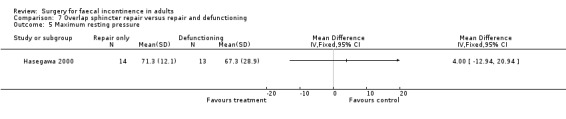
Comparison 7 Overlap sphincter repair versus repair and defunctioning, Outcome 5 Maximum resting pressure.
7.6. Analysis.
Comparison 7 Overlap sphincter repair versus repair and defunctioning, Outcome 6 Maximum squeeze pressure (cm water).
Comparison 8. Overlap sphincter repair versus repair and biofeedback.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients failing to achieve full continence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with no improvement in faecal urgency | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Continence grading scale score at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Patient satisfaction score (VAS) at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Quality of life score ‐ lifestyle at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Quality of life score ‐ Coping at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Quality of life score ‐ (less) depression at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Quality of life score ‐ embarrassment at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Mean resting pressures (cm water) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Mean squeeze pressures (cm water) at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
8.2. Analysis.
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 2 Number of patients failing to achieve full continence.
8.4. Analysis.
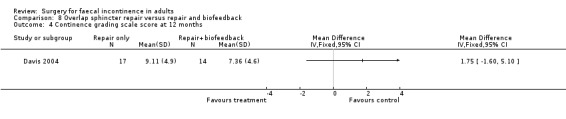
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 4 Continence grading scale score at 12 months.
8.5. Analysis.
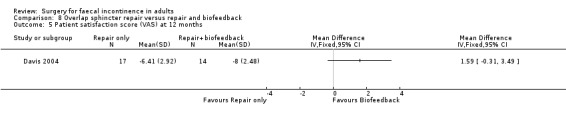
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 5 Patient satisfaction score (VAS) at 12 months.
8.7. Analysis.
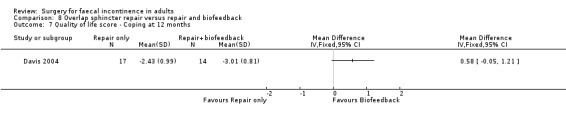
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 7 Quality of life score ‐ Coping at 12 months.
8.10. Analysis.
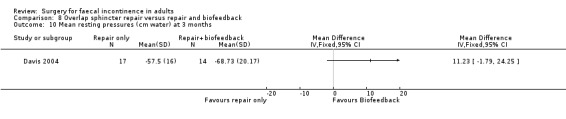
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 10 Mean resting pressures (cm water) at 3 months.
8.11. Analysis.
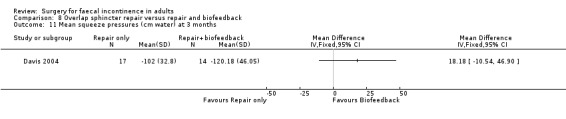
Comparison 8 Overlap sphincter repair versus repair and biofeedback, Outcome 11 Mean squeeze pressures (cm water) at 3 months.
Comparison 9. Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of patients failing to achieve full continence | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Number of patients with no improvement in faecal urgency | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Incontinence score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Maximum resting anal pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Improvement in mucosal electrosensitivity (mA) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Rectal capacity (ml) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Number of patients with no improvement in functional length of anal canal | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9 Number of patients with post‐operative complications | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
9.6. Analysis.
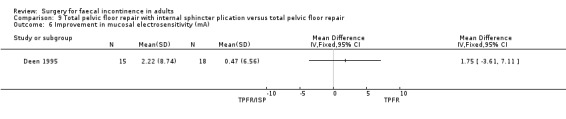
Comparison 9 Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair, Outcome 6 Improvement in mucosal electrosensitivity (mA).
9.7. Analysis.
Comparison 9 Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair, Outcome 7 Rectal capacity (ml).
9.8. Analysis.
Comparison 9 Total pelvic floor repair with internal sphincter plication versus total pelvic floor repair, Outcome 8 Number of patients with no improvement in functional length of anal canal.
Comparison 10. Gluteus maximus transposition (adynamic) versus total pelvic floor repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with no change in incontinence | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of patients failing to achieve full continence | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with no improvement in faecal urgency | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Incontinence score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with post‐operative complications | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Number of patients with adverse effects | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Maximum resting anal pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Mucosal electrosensitivity (mA) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Maximum squeeze pressure (cm/water) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Length of high pressure zone (cm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
10.4. Analysis.
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 4 Incontinence score.
10.5. Analysis.
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 5 Number of patients with post‐operative complications.
10.6. Analysis.
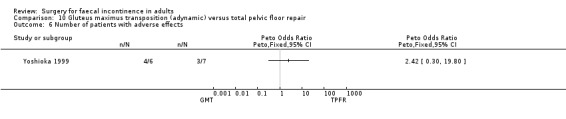
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 6 Number of patients with adverse effects.
10.7. Analysis.
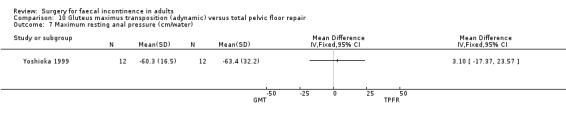
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 7 Maximum resting anal pressure (cm/water).
10.8. Analysis.
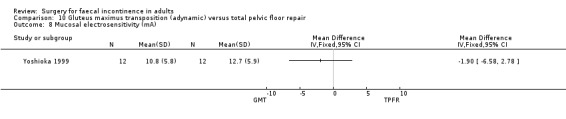
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 8 Mucosal electrosensitivity (mA).
10.9. Analysis.
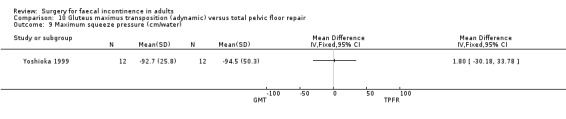
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 9 Maximum squeeze pressure (cm/water).
10.10. Analysis.
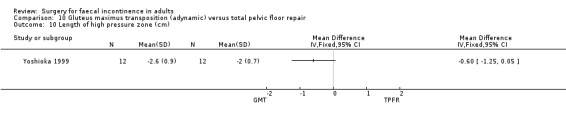
Comparison 10 Gluteus maximus transposition (adynamic) versus total pelvic floor repair, Outcome 10 Length of high pressure zone (cm).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Davis 2004.
| Methods | Allocation: 'random numbers' Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: 4 postponed surgery. 10 declined study. 2 lost to follow‐up Intention‐to‐treat: unclear Follow‐up period: 12 months Setting: single centre, UK Exclusion criteria: prolapse, congenital abnormality, IBD, non‐obstetric trauma, neuropathy, <18 years, planning pregnancy | |
| Participants | Sample size = 31 Mean age = 60 years (range 26‐78 years) Female patients with endosonographically identifiable external sphincter defect | |
| Interventions | Overlap sphincter repair versus sphincter repair and biofeedback | |
| Outcomes | Primary outcomes: ‐ no change in incontinence ‐ failing to achieve full continence Secondary outcomes: ‐ incontinence score ‐ patient satisfaction score ‐ quality of life score (generic) ‐ physiological outcomes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Deen 1993.
| Methods | Allocation: random number tables Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: none Intention‐to‐treat: yes Follow‐up period: 2 years (median) Setting: single centre, United Kingdom Exclusion criteria: patients with co‐morbidity (diabetes) and endosonographic sphincter defect | |
| Participants | Sample size = 36 Mean age = 51 years (range 28‐75 years) Female patients with neuropathic faecal incontinence | |
| Interventions | Anterior levatorplasty versus postanal repair versus total pelvic floor repair | |
| Outcomes | Primary outcomes: ‐ no change in incontinence ‐ failing to achieve full continence Secondary outcomes: ‐ adverse functional effects ‐ physiological outcomes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Deen 1995.
| Methods | Allocation: random number tables Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: none Intention‐to‐treat: yes Follow‐up period (mean): 15.4(±5.5) months for pelvic floor repair alone, 16.8 (±4.5) months for adjuvant internal anal sphincter plication pelvic floor repair group = 15.4 months (SD 5.5) + internal sphincter plication group = 16.8 months (SD 4.5) Setting: single centre, UK Exclusion criteria: patients with co‐morbidity (diabetes) and endosonographic sphincter defect | |
| Participants | Sample size = 30 Mean age 57.5 years (range 27‐72 years) Female patients with neuropathic faecal incontinence | |
| Interventions | Pelvic floor repair versus pelvic floor repair with internal sphincter plication | |
| Outcomes | Primary outcome measures: ‐ none reported Secondary outcome measures: ‐ incontinence scores ‐ physiological outcomes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hasegawa 2000.
| Methods | Allocation: unclear Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: 4 refused to have a stoma. 2 patients given a stoma by the surgeon irrespective of randomisation Intention‐to‐treat: yes Follow‐up period: mean 34 months (range 16‐47 months) Setting: single centre, UK Exclusion criteria: nil | |
| Participants | Sample size = 27 Median age = 48 years (range 23‐76 years) Patients with localised sphincter damage | |
| Interventions | Overlap sphincter repair versus repair and defunctioning | |
| Outcomes | Primary outcome measures: ‐ none reported Secondary outcome measures: ‐ incontinence scores ‐ physiological scores ‐ complications ‐ hospital stay | |
| Notes | Trial closed early due to high complication rate related to the stoma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
O'Brien 2004.
| Methods | Allocation: computer generated random numbers Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: none Intention‐to‐treat: unclear Follow‐up period: 6 months Setting: single centre, Australia Exclusion criteria: patients with previous sphincter repair or endosonographically unrepairable sphincter | |
| Participants | Sample size = 14 Median age = 63 years (range 44‐75 years) Patients with neurogenic faecal incontinence | |
| Interventions | Artificial bowel sphincter versus best supportive care | |
| Outcomes | Primary outcome measures: ‐ none reported Secondary outcomes: ‐ incontinence scores ‐ quality of life scores (generic and specific) ‐ complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Osterberg 2004.
| Methods | Allocation: random block Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: 11 (7 from control group) Intention‐to‐treat: unclear Follow‐up period: 2 years Setting: single centre, Sweden Exclusion criteria: patients with an endosonographic sphincter defect, rectal prolapse or intra‐anal intussusception | |
| Participants | Sample size = 59 Median age = 66 years (range 43‐81 years) Patients with neuropathic faecal incontinence | |
| Interventions | Anterior levatoroplasty versus anal plug stimulation | |
| Outcomes | Primary outcomes: ‐ no change in incontinence Secondary outcomes: ‐ use of pads ‐ complications ‐ physiological outcomes ‐ incontinence score ‐ patient assessment of handicap | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Tjandra 2003.
| Methods | Allocation: unclear Blinding: participants blinded to repair. No mention of blinding of surgeons or outcome assessors Withdrawals: none Intention‐to‐treat: yes Follow‐up period: median 18 months (range 10‐34 months) Setting: single centre, Australia Exclusion criteria: patients with prolonged pudendal nerve terminal motor latency | |
| Participants | Sample size = 23 Median age = 46 years (range 31‐71 years) Women with localised anterior defect of external sphincter after obstetric damage | |
| Interventions | Overlap sphincter repair versus direct end to end repair | |
| Outcomes | Primary outcomes: ‐ no change in incontinence Secondary outcomes: ‐ adverse function ‐ complications ‐ physiological outcomes ‐ incontinence score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
van Tets 1998.
| Methods | Allocation: random lots Blinding: no mention of blinding for participants, surgeons or outcome assessors Withdrawals: none Intention‐to‐treat: yes Follow‐up period, mean 3.5 years (range 1.5‐5) Setting: single centre, the Netherlands Exclusion criteria: patients with endosonographic sphincter defect | |
| Participants | Sample size = 20 Mean age 55 year (range 34‐74 years) Female patients with neuropathic faecal incontinence |
|
| Interventions | Postanal repair versus total pelvic floor repair | |
| Outcomes | Primary outcome measures: ‐ no change in incontinence Secondary outcome measures: ‐ physiological outcomes | |
| Notes | Authors contacted regarding details of randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Yoshioka 1999.
| Methods | Allocation: random block Blinding: no mention of blinding of participants, surgeons or outcome assessors Withdrawals: none Intention‐to‐treat: yes Follow‐up period: 18 months Setting: single centre, UK Exclusion criteria: patients with co‐morbidity (diabetes) and endosonographic sphincter defect | |
| Participants | Sample size = 24 Mean age = 60 years (range 30‐77 years) Female patients with neuropathic faecal incontinence | |
| Interventions | Total pelvic floor repair versus gluteus maximus transposition (without electrical stimulation) | |
| Outcomes | Primary outcome: ‐ failing to achieve full continence ‐ faecal urgency Secondary outcome measures: ‐ post‐operative complications ‐ adverse functional effects ‐ physiological outcomes | |
| Notes | Pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dehli 2013 | Study looking at bulking agents. Relevant to another review |
| Farrell 2010 | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
| Farrell 2012 | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
| Fernando 2006a | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
| Fitzpatrick 2000 | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
| Garcia V 2005 | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
| Graf 2011 | Study looking at bulking agents. Relevant to another review |
| Leroi 2005 | Sacral nerve stimulation is analysed in another Cochrane review |
| Loder 1993 | No RCT or clinical trial. Letter published in a journal |
| Maeda 2007 | Study looking at bulking agents. Relevant to another review |
| Michelsen 2008 | Sacral nerve stimulation is analysed in another Cochrane review |
| Nordenstam 2008 | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
| Rygh 2010 | Study looking at bulking agents. Relevant to another review |
| Siphrouis 2004 | Study looking at bulking agents. Relevant to another review |
| Tjandra 2004 | Study looking at bulking agents. Relevant to another review |
| Tjandra 2008 | Sacral nerve stimulation is analysed in another Cochrane review |
| Tjandra 2009 | Study looking at bulking agents. Relevant to another review |
| Vaizey 1999 | Sacral nerve stimulation is analysed in another Cochrane review |
| Williams 2004 | Study looking at acute obstetric injury repair. Patients not incontinent before repair |
Contributions of authors
One review author (P Bachoo) wrote the initial protocol of the review. Two review authors (P Bachoo, M Brazzelli) assessed the pertinence and quality of included studies and independently extracted data from trials reports. All review authors (P Bachoo, M Brazzelli, A Grant) interpreted the results and contributed to the writing of the initial version of the review. With regard to the updates, three review authors (SR Brown, H Wadhawan, R Nelson) assessed the appropriateness and quality of the further studies, interpreted the results and contributed to the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS National R&D Programme for People with Physical and Complex Disabilities, UK.
Chief Scientist Office, Scottish Executive Health Department, UK.
-
The National Institute for Health Research, UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Davis 2004 {published data only}
- Davis KJ, Kumar D, Poloniecki J. Adjuvant biofeedback following anal sphincter repair: a randomized study. Alimentary Pharmacology & Therapy 2004;20:539‐49. [DOI] [PubMed] [Google Scholar]
Deen 1993 {published data only}
- Deen KI, Oya M, Oritz J, Keighley MRB. Randomized trial comparing three forms of pelvic floor repair for neuropathic faecal incontinence. British Journal of Surgery 1993;80:794‐8. [DOI] [PubMed] [Google Scholar]
Deen 1995 {published data only}
- Deen KI, Kumar D, Williams JG, Grant EA, Keighley MRB. Randomized trial of internal anal sphincter plication with pelvic floor repair for neuropathic fecal incontinence. Diseases of the Colon and Rectum 1995;38(1):14‐8. [DOI] [PubMed] [Google Scholar]
Hasegawa 2000 {published data only}
- Hasegawa H, Yoshioka K, Keighley MRB. Randomized trial of fecal diversion for sphincter repair. Disease of the Colon and Rectum 2000;43:961‐5. [DOI] [PubMed] [Google Scholar]
O'Brien 2004 {published data only}
- O'Brien PE, Dixon JB, Skinner S, Laurie C, Khera A, Fonda D. A prospective randomized controlled clinical trial of placement of the artificial bowel sphincter (Acticon neosphincter) for the control of fecal incontinence. Disease of the Colon and Rectum 2004;47:1852‐60. [DOI] [PubMed] [Google Scholar]
Osterberg 2004 {published data only}
- Osterberg A, Edebol Eeg‐Olofsson K, Hallden M, Graf W. Randomized clinical trial comparing conservative and surgical treatment of neurogenic faecal incontinence. British Journal of Surgery 2004;91:1131‐7. [DOI] [PubMed] [Google Scholar]
Tjandra 2003 {published data only}
- Tjandra JJ, Han WR, Goh J, Carey M, Dwyer P. Direct repair versus overlapping sphincter repair. A randomised controlled trial. Diseases of the Colon and Rectum 2003;46:937‐43. [DOI] [PubMed] [Google Scholar]
van Tets 1998 {published data only}
- Tets WF, Kuijpers JHC. Pelvic floor procedures produce no consistent changes in anatomy or physiology. Diseases of the Colon and Rectum 1998;41(3):365‐9. [DOI] [PubMed] [Google Scholar]
Yoshioka 1999 {published data only}
- Yoshioka K, Ogunbiyi OA, Keighley MRB. Pilot study of total pelvic floor repair or Gluteus Maximus transposition for postobstetric neuropathic fecal incontinence. Diseases of the Colon and Rectum 1999;42(2):252‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dehli 2013 {published data only}
- Delhi T, Stordahl A, Vatten LJ, Romundstad PR, Mevik K, Sahlin Y, et al. Sphincter training or anal injections of dextranomer for treatment of anal incontinence: a randomised controlled trial. Scandinavian Journal of Gastroenterology 2013;48(3):302‐10. [DOI] [PubMed] [Google Scholar]
Farrell 2010 {published data only}
- Farrell SA, Gilmour D, Turnbull GK, Schmidt MH, Baskett TF, Flowerdew G, Fanning CA. Overlapping compared with end‐to‐end repair of third‐ and fourth‐degree obstetric anal sphincter tears: a randomised controlled trial. Obstetrics and Gynecology 2010;116(1):16‐24. [DOI] [PubMed] [Google Scholar]
Farrell 2012 {published data only}
- Farrell SA, Flowerdew G, Gilmour D, Turnbull GK, Schmidt MH, Baskett TF, Fanning CA. Overlapping compared with end‐to‐end repair of complete third‐degree or fourth‐degree obstetric tears: three‐year follow up of a randomised controlled trial.. Obstetrics and Gynecology 2012;120(4):803‐8. [DOI] [PubMed] [Google Scholar]
Fernando 2006a {published data only}
- Fernando R, Sultan AH, Kettle C, Radley S, Jones P, O'Brien S. A randomised trial of overlap vs end‐to‐end primary repair of the anal sphincter (abstract). Neurourology and Urodynamics 2004;23(5/6):411‐2. [Google Scholar]
- Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O'Brien PM. Repair techniques for obstetric anal sphincter injuries: a randomized controlled trial. Obstetrics and Gynecology 2006;107(6):1261‐8. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2000 {published data only}
- Fitzpatrick M, Behan M, O'Connell R, O'Herlihy C. A randomised clinical trial comparing primary overlap with approximation repair of third‐degree tears. American Journal of Obstetrics and Gynecology 2000;183:1220‐4. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick M, Cassidy M, Behan M, O'Herlihy C, O'Connell PR. Primary anal sphincter repair: influence of technique of repair (abstract). Colorectal Disease 1999;1 Suppl 1:10. [Google Scholar]
Garcia V 2005 {published data only}
- Garcia V, Rogers RG, Kim SS, Hall RJ, Kammerer‐Doak DN. Primary repair of obstetric anal sphincter laceration: A randomised trial of two surgical techniques. American Journal of Obstetrics and Gynecology 2005;192:1697‐701. [DOI] [PubMed] [Google Scholar]
Graf 2011 {published data only}
- Graf W, Mellgren A, Matzel KE, Hull T, Johansson C, Bernstein M, NASHA Dx Study Group. Efficacy of dextranomer in stabilised hyaluronic acid for the treatment of faecal incontinence: a randomised, sham‐controlled trial. Lancet 2011;377(9770):997‐1003. [DOI] [PubMed] [Google Scholar]
Leroi 2005 {published data only}
- Leroi AM, Parc Y, Lehur PA, Mion F, Barth X, Rullier E, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double‐blind crossover study. Annals of Surgery 2005;242(5):662‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loder 1993 {published data only}
- Loder PB, Phillips RK. Randomized trial comparing three forms of pelvic floor repair for neuropathic faecal incontinence. British Journal of Surgery 1993;80(10):1352. [DOI] [PubMed] [Google Scholar]
Maeda 2007 {published data only}
- Maeda Y, Vaisey CJ, Kamm MA. Pilot study of two new injectable bulking agents for the treatment of faecal incontinence. Colorectal Disease 2007;10(3):268‐72. [DOI] [PubMed] [Google Scholar]
Michelsen 2008 {published data only}
- Michelsen HB, Krogh K, Buntzen S, Laurberg S. A prospective randomised study: switch off the sacral nerve stimulator during the night?. Diseases of the Colon and Rectum 2008;51(5):538‐40. [DOI] [PubMed] [Google Scholar]
Nordenstam 2008 {published data only}
- Nordenstam J, Mellgren A, Altman D, López A, Johansson C, Anzén B, et al. Immediate or delayed repair of obstetric anal sphincter tears ‐ a randomised controlled trial. BJOG 2008;115(7):857‐65. [DOI] [PubMed] [Google Scholar]
Rygh 2010 {published data only}
- Rygh AB, Korner H. The overlap technique versus end‐to‐end approximation technique for primary repair of obstetric anal sphincter rupture: a randomised controlled study. Acta Obstetricia et Gynecologica Scandinavica 2010;89(10):1256‐62. [DOI] [PubMed] [Google Scholar]
Siphrouis 2004 {published data only}
- Siproudhis L, Morcet J, Laine F. Elastomer implants in faecal incontinence: a blind, randomised placebo‐controlled study. Alimentary Pharmacology and Therapeutics 2007;25(9):1125‐32. [DOI] [PubMed] [Google Scholar]
Tjandra 2004 {published data only}
- Tjandra JJ, Lim JF, Hiscock R, Rajendra P. Injectable silicone biomaterial for faecal incontinence caused by internal anal sphincter dysfunction is effective. Diseases of the Colon and Rectum 2004;47(12):2138‐46. [DOI] [PubMed] [Google Scholar]
Tjandra 2008 {published data only}
- Tjandra JJ, Chan MK, Yeh CH, Murray‐Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe faecal incontinence: a randomised controlled trial. Diseases of the Colon and Rectum 2008;51(5):494‐502. [DOI] [PubMed] [Google Scholar]
Tjandra 2009 {published data only}
- Tjandra JJ, Chan MK, Yeh HC. Injectable silicone biomaterial (PTQ) is more effective than carbon‐coated beads (Durasphere) in treating passive faecal incontinence ‐ a randomized controlled trial. Colorectal Disease 2009;11(4):382‐9. [DOI] [PubMed] [Google Scholar]
Vaizey 1999 {published data only}
- Vaizey CJ, Kamm MA, Roy AJ, Nicholls RJ. Double blind crossover study of sacral nerve stimulation for fecal incontinence. Diseases of the Colon and Rectum 1999;43:298‐302. [DOI] [PubMed] [Google Scholar]
Williams 2004 {published data only}
- Williams A, Adams E, Tincello D, Alfirevic Z, Richmond D. Randomised controlled trial of third degree perineal tear: medium term outcome (abstract). Proceedings of the International Continence Society UK 11th annual scientific meeting. 2004:33‐4. [17174]
Additional references
Andrews 1964
- Andrews J, Nathan P. Lesions of the anterior frontal lobes and disturbances of micturition and defecation. Brain 1964;87:233‐62. [DOI] [PubMed] [Google Scholar]
Burnstock 1990
- Burnstock G. Innervation of bladder and bowel. Ciba Foundation Symposium 1990;151:2‐18, 18‐26. [DOI] [PubMed] [Google Scholar]
Byar 1976
- Byar DP, Simon RM, Friedewald WT, Schlesselman JJ, DeMets DL, Ellenberg JH, et al. Randomized clinical trials. Perspectives on some recent ideas. New England Journal of Medicine 1976;295(2):74‐80. [DOI] [PubMed] [Google Scholar]
Chen 1998
- Chen ASH, Luchtefeld MA, Senagore AJ, Mackeigan JM, Hoyt C. Pudendal nerve latency. Does it predict outcome of anal sphincter repair. Diseases of the Colon and Rectum 1998;41(8):1005‐9. [DOI] [PubMed] [Google Scholar]
Counihan 2005
- Counihan TC, Madoff RD. Faecal incontinence. In: Fazio VW, Church JM, Delaney CP editor(s). Current therapy in Colon and Rectal Surgery. 2nd Edition. Philadelphia: Elsevier Mosby, 2005:105‐12. [Google Scholar]
de Groat 1990
- Groat WC. Central neural control of the lower urinary tract. Ciba Foundation Symposium 1990;151:27‐44, 44‐56. [DOI] [PubMed] [Google Scholar]
EUCTR 2010
- Anonymous. Skeletal muscle‐derived cell implantation for the treatment of fecal incontinence: a multicentre, randomized, double‐blind, placebo controlled, parallel, dose‐finding clinical study . EU Clinical Trials Register 2011. [EUCTR2010‐021463‐32‐AT]
Felt‐Bersma 1997
- Felt‐Bersma RJF, Poen AC, Cuesta MA, Meuwissen SGM. Anal sensitivity test; what does it measure and do we need it. Cause or derivative of anorectal complaints. Diseases of the Colon and Rectum 1997;40(7):811‐6. [DOI] [PubMed] [Google Scholar]
Fernando 2006b
- Fernando RJ, Sultan AHH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002866.pub2] [DOI] [PubMed] [Google Scholar]
Ghahramani 2013
- Ghahramani. Evaluation of efficacy of preoperative biofeedback therapy before sphincteroplasty ‐ levatorplasty for fecal incontinence management due to obstetric trauma. Iranian Registry of Clinical Trials 2013.
Guttierez 2004
- Guttierez AB, Madoff RD, Lowry AC, Parker SC, Buie WD, Baxter NN. Long‐term results of anterior sphincteroplasty. Disease of the Colon and Rectum 2004;47:727‐32. [DOI] [PubMed] [Google Scholar]
Halverson 2002
- Halverson AJ, Hull TL. Long‐term outcome of overlapping anal sphincter repair. Diseases of the Colon and Rectum 2002;45:345‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S. [Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008].]. In: Higgins JPT, Green S editor(s). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. Oxford: Update Software, 2008. [Google Scholar]
Integrated continence service 2000
- Bladder and Bowel Foundation. Making the case for an integrated continence service. Bladder and Bowel Foundation 2000.
ISRCTN88559475
- Horrocks E, Knowles C. Control of faecal incontinence using distal neuromodulation. ISRCTN Register 2011. [ISRCTN8855975]
Johanson 1996
- Johanson JF, Lafferty J. Epidemiology of faecal incontinence: the silent affliction. American Journal of Gastroenterology 1996;91(1):33‐6. [PubMed] [Google Scholar]
Kalantar 2002
- Kalantar JS, Howell S, Talley NJ. Prevalence of faecal incontinence and associated risk factors; an under‐diagnosed problem in the Australian community?. Medical Journal of Australia 2002;176:54‐7. [PubMed] [Google Scholar]
Kamm 1998
- Kamm MA. Faecal incontinence. BMJ 1998;316(7130):528‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keating 1997
- Keating JP, Stewart PJ, Eyers AA, Warner D, Bokey EL. Are special investigations of value in the management of patients with fecal incontinence?. Diseases of the Colon and Rectum 1997;40(8):896‐901. [DOI] [PubMed] [Google Scholar]
Lam 1999
- Lam TCF, Kennedy ML, Chen FC, et al. Prevalence of Faecal incontinence: obstetric and constipation‐related risk factors; a population‐based study. Colorectal Disease 1999;1:197‐203. [DOI] [PubMed] [Google Scholar]
MacArthur 1997
- MacArthur C, Bick DE, Keighley MR. Faecal incontinence after childbirth. British Journal of Obstetrics and Gynaecology 1997;104:46‐50. [DOI] [PubMed] [Google Scholar]
Maeda 2009
- Maeda Y, Laurberg S, Norton C. Perianal injectable bulking agents as treatment for faecal incontinence in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007959.pub3] [DOI] [PubMed] [Google Scholar]
Malouf 2000
- Malouf AJ, Norton CS, Engel AF, Nicholls RJ, Kamm MA. Long‐term results of overlapping anterior anal‐sphincter repair for obstetrical trauma. Lancet 2000;355:260‐5. [DOI] [PubMed] [Google Scholar]
Mellgren 1999
- Mellgren A, Jenson LL, et al. Long‐term cost of fecal incontinence secondary to obstetric injuries. Diseases of the Colon and Rectum 1999;42(7):857‐65. [DOI] [PubMed] [Google Scholar]
Michot 2012
- Michot F. Myoblast for anal incontinence. ClinicalTrials.gov 2012. [NCT01523522]
Mowatt 2007
- Mowatt G, Glazener CMA, Jarrett M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004464.pub2] [DOI] [PubMed] [Google Scholar]
Nakayama 1997
- Nakayama H, Jorgensen HS, Pederson PM, Raaschou HO, Olsen TS. Prevalence and risk factors of Incontinence after stroke. The Copenhagen stroke study. Stroke 1997;28(1):58‐62. [DOI] [PubMed] [Google Scholar]
NCT01044589 2010
- Anonymous. Comparison of the efficacy of a biological implant to reinforce overlapping sphincter repair versus overlapping sphincter repair alone. ClinicalTrials.gov 2010. [NCT01044589]
Nelson 1995
- Nelson R, Norton N, Cautley E, Furner F. Community‐based prevalence of anal incontinence. JAMA 1995;274(7):559‐61. [PubMed] [Google Scholar]
Norderval 2012
- Norderval S, Rydningen M. A blinded randomized controlled clinical trial comparing sacral nerve modulation and anal bulking injections as treatment for fecal incontinence after obstetric anal sphincter injuries (OASIS). ClinicalTrials.gov 2012. [NCT01528995]
Parellada 1998
- Parellada CM, Miller AS, Williamson MER, Johnston D. Paradoxical high anal resting pressures in men with idiopathic fecal seepage. Diseases of the Colon and Rectum 1998;41(5):593‐7. [DOI] [PubMed] [Google Scholar]
Parks 1975
- Parks AG. Anorectal incontinence. Proceedings of the Royal Society of Medicine 1975;68:681‐90. [PMC free article] [PubMed] [Google Scholar]
Perry 2002
- Perry S, Shaw C, Varma MG, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002;50:480‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pescatori 2004
- Pescatori M. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. British Journal of Surgery 2004;91:1559‐69. [DOI] [PubMed] [Google Scholar]
Pfeifer 1997
- Pfeifer J, Salanga VD, Agachan F, Weiss EG, Wexner SD. Variation in pudendal nerve terminal motor latency according to disease. Diseases of the Colon and Rectum 1997;40(1):79‐83. [DOI] [PubMed] [Google Scholar]
Rainey 1990
- Rainey JB, Donaldson DR, Thompson JPS. Postanal repair: which patients derive most benefit?. Journal of the Royal College of Surgeons of Edinburgh 1990;35:101‐5. [PubMed] [Google Scholar]
Rockwood 2000
- Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrontonis SC, Thorson AG, et al. Fecal incontinence quality of life scale: quality of life instrument for patients with fecal incontinence. Diseases of the Colon and Rectum 2000;43:9‐16. [DOI] [PubMed] [Google Scholar]
Ryhammer 1997
- Ryhammer AM, Laurberg S, Hermann AP. Test‐retest repeatability of anorectal physiology tests in healthy volunteers. Diseases of the Colon and Rectum 1997;40(3):287‐92. [DOI] [PubMed] [Google Scholar]
Setti Carraro 1994
- Setti Carraro P, Kamm MA, Nicholls RJ. Long‐term results of postanal repair of neurogenic faecal incontinence. British Journal of Surgery 1994;81(1):140‐4. [DOI] [PubMed] [Google Scholar]
Siproudhis 2006
- Siproudhis L, Pigot F, Godeberge P, et al. Defecation disorders: A French population survey. Diseases of the Colon and Rectum 2006;49:219‐27. [DOI] [PubMed] [Google Scholar]
Sun 1990
- Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with fecal incontinence. Gut 1990;31(9):1056‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2002
- Takahashi T, Garcia‐Osogobio S, Valdovinos MA, Mass W, Jimenez R, Jauregui LA, et al. Radio‐frequency energy delivery to the anal canal for the treatment of fecal incontinence. Diseases of the Colon and Rectum 2002;45:915‐22. [DOI] [PubMed] [Google Scholar]
Tou 2008
- Tou S, Brown SR, Malik AI, Nelson RL. Surgery for complete rectal prolapse in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001758.pub2] [DOI] [PubMed] [Google Scholar]
Turnberg 1995
- Turnberg L, Brocklehurst J, Fowler C, Borzyskowski M, Cardozo L, Chellingsworth M, et al. Incontinence. Causes, management and provision of services. May. London: The Royal College of Physicians, 1995. [Google Scholar]
Uher 1998
- Uher EM, Swash M. Sacral reflexes physiology and clinical application. Diseases of the Colon and Rectum 1998;41(9):1165‐77. [DOI] [PubMed] [Google Scholar]
Vaizey 1998
- Vaizey CJ, Kamm MA, Nicholls RJ. Recent advances in the surgical treatment of faecal incontinence. British Journal of Surgery 85;5:596‐603. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey Manual and Interpretation Guide. Boston: New England Medical Centre, 1993. [Google Scholar]
Whitehead 2009
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology 2009;137(2):512‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2011
- Wong MTC, Meurette G, Stanherlin P, Lehur PA. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence . Diseases of the Colon and Rectum 2011;54:773‐9. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]


