Abstract
Purpose
The management of the tuberous breast deformity in the female patient is well described. However, the presence of this variant in male patients is particularly rare, and few reports on the management of this condition are available.
Case presentation
A 12-year-old prepubescent male with bilateral gynecomastia and tuberous breast deformities was referred to our department for treatment. Our surgical management, including free nipple areolar complex harvest, mastectomy, removal of excess skin and subsequent nipple grafting, is presented in detail. We observed a cosmetically acceptable result with restoration of a masculine-appearing nipple-areolar complex and good patient satisfaction at 6-month follow-up.
Conclusions
Tuberous breast deformities in male patients are rare. Our treatment of a prepubertal male patient with this deformity using mastectomies and free nipple areolar complex grafting provided a cosmetically acceptable result. Here, we review the current literature on tuberous breast deformities in males and describe our approach to treatment.
Keywords: Tuberous, Breast, Gynecomastia, Male, Nipple, Pediatric
Introduction
Pediatric breast anomalies represent a broad spectrum of disorders that can confer significant psychological and psychosocial burdens upon both patient and family.1–6 Gynecomastia is amongst the most common benign condition of the male breast, affecting up to 65% of adolescents.2, 7 Development of gynecomastia is generally attributed to imbalanced estrogen: androgen ratios during hormonal axis maturation in puberty.2, 3, 5 Though typically self- resolving within 1–2 years, persistent gynecomastia beyond one year is associated with hyalinising fibrotic changes that portend a higher incidence of surgical necessity.1, 2, 3,7
A particularly rare variant of gynecomastia is the tuberous male breast. Features include a horizontally- and vertically-constricted breast base, enlarged nipple-areolar complex, deficient skin envelope, inframammary fold malposition, and apparent parenchymal herniation into the areola. First described by Rees and Ashton in 19768, this clinical entity is characterized by a variety of names (Snoopy deformity, herniated areolar complex, constricted breasts, etc.) and classification schemes.5, 6,9, 10, 11 The exact etiology of tuberous breast deformity remains a subject of considerable speculation with hypotheses ranging from anomalous annular thickening of the superficial fascia preventing radial glandular expansion to a genetically-mediated disorder of collagen deposition.5, 6, 12, 13 Consequently, myriad surgical strategies have been described, each of which attempts to address the perceived underlying pathophysiologic basis of the malformation while optimising scar burden, function, and aesthetic result.5, 6, 12, 13
Though well described in females, much less has been written about the tuberous breast deformity in males. Further, the majority of these articles describe tuberous breasts in pubertal males, a developmental stage where gynecomastia is common. In contrast, prepubertal gynecomastia (i.e. breast enlargement in the absence of other signs of pubertal development) is much rarer.5, 14 It generally occurs secondary to underlying disease (e.g. malignancy, congenital adrenal hyperplasia) or medications (e.g. antipsychotics) with corresponding laboratory evidence of hormonal irregularity. However, prepubertal gynecomastia can occasionally be idiopathic. We describe our surgical approach for a high stage idiopathic tuberous breast deformity in a prepubescent male patient.
Case report
A 12-year-old male presented with a two-year history of progressive, worsening bilateral gynecomastia. Though he denied being teased, he did endorse feeling embarrassed in public and needing to hunch his shoulders to conceal his breasts. He was otherwise healthy and not taking any medications. The patient's father and a cousin both had history of gynecomastia, for which the cousin underwent surgery.
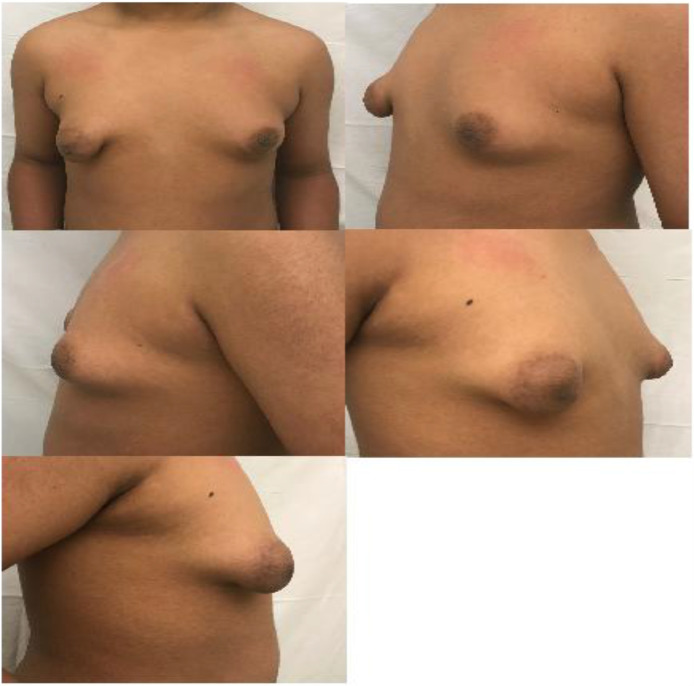
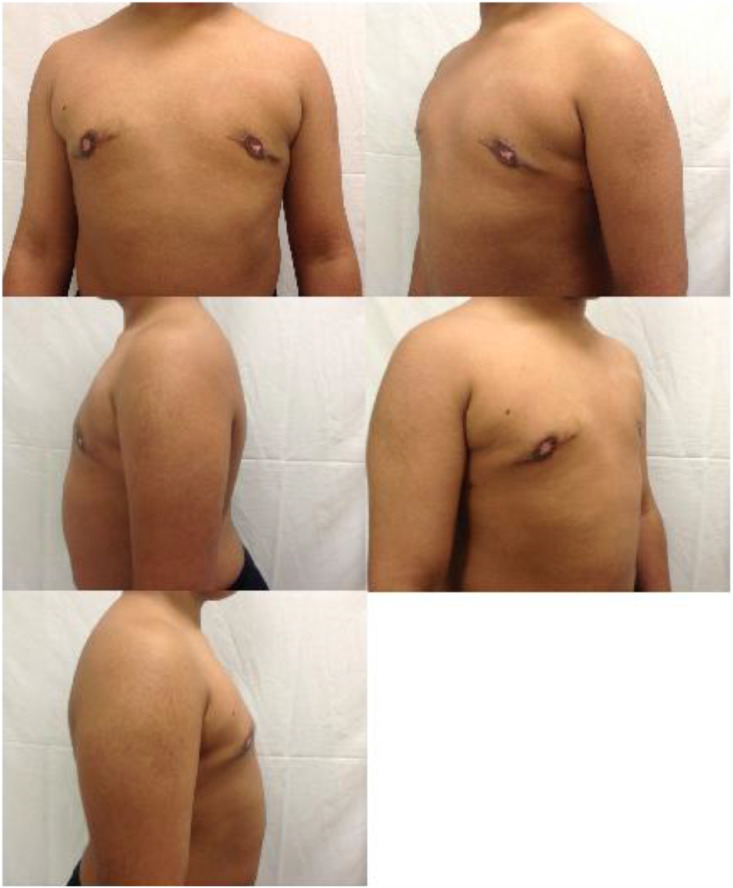
On preoperative physical exam (Figure 1) the patient appeared his stated age, but with poor posture. His height and weight were in the 60th, 90th percentiles, respectively. Body mass index (BMI) was 25 kg/m2. He had bilateral gynecomastia with widened nipple-areolar complexes and total base deficits consistent with Grolleau type III/Heimburg type IV tubular breast deformity, with particular right-sided severity. Pubic hair and genitalia were Tanner stage I. There were no axillary masses, nipple retraction, or nipple discharge.
Figure 1.
Preoperative patient photographs. A prepubescent, 12-year-old male with bilateral gynecomastia and tuberous breast deformity is shown in the frontal (a), left oblique (b), left lateral (c), right oblique (d), and right lateral (e) positions.
Laboratory studies, including testosterone (374 ng/dL), dehydroepiandrosterone sulfate (49 µg/dL), prolactin (9.1 ng/dL), estradiol (34 pg/dL), TSH (2.02 uIU/mL), free T4 (1.09 ng/dL), human chorionic gonadotropin (<1 mIU/mL), lipid panel, complete blood count, serum chemistry, and liver function tests were within reference ranges.
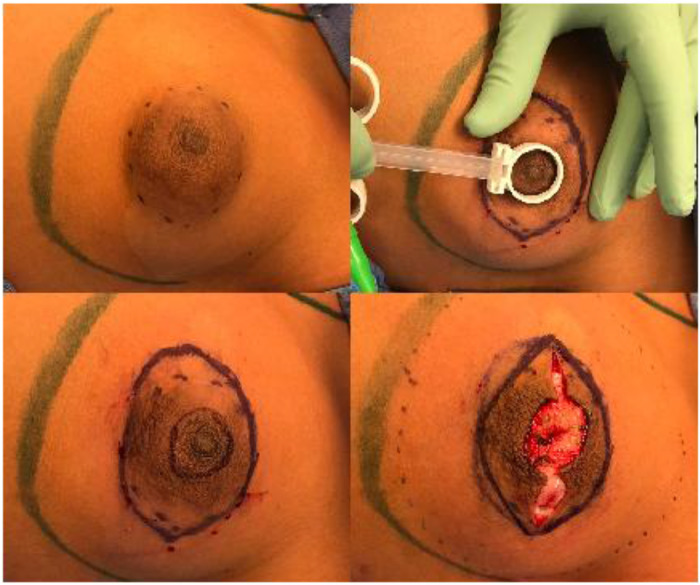
After discussion with the patient and his parents, bilateral mastectomies with free nipple grafting were performed. The periphery of the overdeveloped areolae was marked and the nipple-areolar complexes were resized to 20 mm using the plunger flange of a syringe (Figure 2a and b). Elliptical markings were made incorporating the nipple-areolar complex (Figure 2c). Marcaine with 1:200,000 epinephrine was injected, intra-areolar incisions made, and the new nipple-areola complexes harvested as full thickness grafts (Figure 2d).
Figure 2.
Marking and harvesting of the male nipple areolar complex. (a) The periphery of the overdeveloped areolae was marked. (b) Nipple-areolar complexes were resized to 20 mm using the plunger flange of a syringe. (c) Elliptical markings were made incorporating the native nipple areolar complex. (d) Intra-areolar incision and harvest of new nipple-areola complex as full thickness graft (Figure 2d).
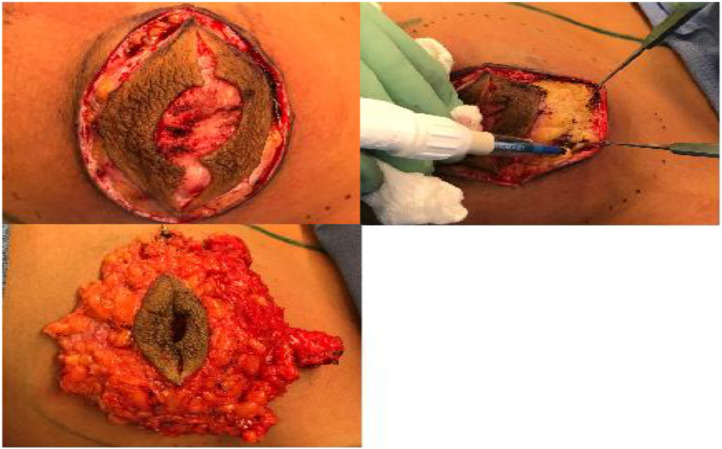
Superior, medial, lateral, and inferior mastectomy flaps were developed (Figure 3a and b) and the conically-shaped breast tissue was excised at the level of the pre-pectoralis fascia fat, yielding 140 g from the left breast and 130 g from the right (Figure 3c). Blake drains were placed bilaterally, and three-layered closure was performed.
Figure 3.
Mastectomy for gynecomastia. (a) Elliptical incision incorporating the native nipple areolar complex. (b) Development of mastectomy flaps. (c) Excised breast tissue and overlying nipple areolar complex placed over breast skin.
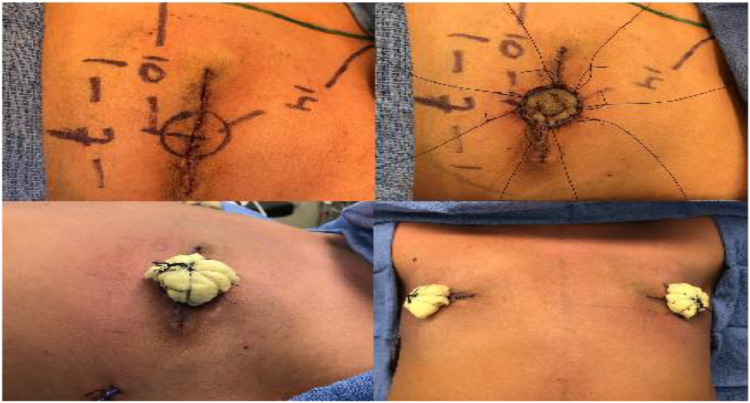
The new bilateral nipple-areolar locations were then positioned just medial to the inferolateral border of the pectoralis muscle, with a 14 cm sternal notch-to-nipple distance and 10 cm medial-to-lateral sternal distance (Figure 4a). The free nipple grafts were then inset onto the de-epithelialised recipient sites using half-buried horizontal mattress sutures (Figure 4b) and a bolster dressing was secured (Figure 4c and d). The patient's chest was then wrapped in a compressive vest, which he was encouraged to wear as frequently as possible.
Figure 4.
Construction of new nipple-areolar complex with free nipple grafts. (a) Markings and measurements for the free nipple grafts. Grafts were placed just medial to the inferolateral border of the pectoralis muscle with a sternal notch-to-nipple distance of 14 cm and 10 cm lateral to the sternal midline bilaterally. (b) Inset of the free nipple grafts onto de-epithelialised recipient sites using half-buried horizontal mattress sutures. (c) Bolsters of sterilised cotton balls wrapped in xeroform and bacitracin applied and secured with silk sutures. (d) Final appearance of chest with bolsters in place.
Histological examination of the breast specimens revealed adipose and prominent dense gray- white fibrous tissue, consistent with true gynecomastia (versus the adipose-dominant appearance of pseudo-gynecomastia). There were no other abnormal findings. At 6-month's follow-up, save for hypopigmentation at the centre of the nipples, the patient had a cosmetically acceptable result (Figure 5). Both patient and his father reported satisfaction with the outcome.
Figure 5.
Postoperative patient photographs. 6-month follow-up views in the frontal (a), left oblique (b), left lateral (c), right oblique (d), and right lateral (e) positions.
Discussion
The tuberous breast has an unmistakably characteristic appearance, but the epidemiology, classification systems, surgical methods, and etiology surrounding this unique breast-shape deformity are more nebulous. The exact incidence of male tuberous breast deformity is unknown, but thought to be rare.1, 4, 6, 15 In classifying tuberous breast deformity, von Heimburg described a type I deformity as a hypoplastic lower medial quadrant, type II as a hypoplastic lower medial and lateral quadrant with sufficient subareolar skin, type III as hypoplasia of the lower medial and lateral quadrants with subareolar skin deficiency, and type IV as severe breast constriction with minimal breast base.9 Grolleau et al.10 simplified this system by effectively combining the Heimberg type II and type III classifications, arguing that no objective anatomic or clinical differences differentiated the two. Most recently, Costagliola et al.6 proposed a “completed” classification scheme, designating type 0 as one in which isolated nipple-areolar complex herniation occurs in the setting of a normal mammary base. Common to these systems is the need to tailor surgical considerations based on the degree of base constriction, sufficiency of skin envelope, inframammary fold position, breast volume, and nipple position.
Surgical correction of the male tuberous breast deformity aims to achieve a cosmetically acceptable result with restoration of a masculine-appearing nipple-areolar complex. Additionally, residual skin redundancy following tissue excision must be addressed.1, 4 As such, surgical intervention is highly dependent on the geometry and type of deformity and must be individualised. Previous approaches to this problem are summarised in Table 1. For the type 0 male tuberous breast, Godwin utilises periareolar “doughnut” incisions with a superiorly-based dermoglandular pedicle, lower breast bud reduction, and careful radial scoring of the undersurface of the nipple-areolar complex.15 Hamilton described two techniques for more severe types, including a Wise-pattern reduction with free nipple grafting in a patient with significant skin excess, and a four-pedicled circumareolar approach with inter-pedicle excision and liposuction.1 Monteiro et al.4 approached a Heimberg type IV male tuberous breast via a circumareolar incision with a superomedial pedicle in conjunction with liposuction. They also emphasise retaining a layer of fat above the pectoralis fascia to avoid cutaneous depression. Other techniques, such as inferior pedicle reduction mammoplasty, have also been described.2
Table 1.
Literature review: previously reported treatments for male tuberous breasts.
| Title | Authors | Published Year | No. patients | Age, y | Treatment Modalities | Follow-up | Remarks |
|---|---|---|---|---|---|---|---|
| The Tuberous | Hamilton et al.1 | 2003 | 2 | 15,15 | Patient 1: Wise reduction pattern and free nipple grafts | N/A | Patient 1: mild hypertrophy of ‘inframammary’ scars. Patient 2: Uneventful recovery |
| Male Breast | Patient 2: Circumferential areola- reducing approach. De-epithelialization of excess areola. Liposuction at periphery of gland. Nipple vascularized on four dermal pedicles at 3-, 6-, 9- and 12- o'clock. Dermis and glandular tissue between pedicles removed. | ||||||
| Gynecomastia And Tuberous Breast: Assessment and Surgical Approach | Klinger et al.12 | 2009 | 6 | N/A | Liposuction by tumescent technique, skin and gland excess excision and gland redraping | 1 y | Liposuction using 2-mm blunt cannula, concentric circle around areola deepithelized; semicircular infra-areolar incision of dermis with superior dermal pedicle to the NAC; release of constricted base with cautery. Radial incisions of residual breast fibrous tissue |
| Tuberous Male Breast: Assessment and Esthetic Correction | Monteiro et al.4 | 2015 | 1 | 15 | Liposuction by tumescent technique, skin and gland excess excision and gland redraping | 6 m | Liposuction with 4-mm blunt cannula, incision at periareola, excess skin deepithelized, nipple on superomedial dermal pedicle; base released with electrocautery and radial incisions of the residual breast fibrous tissue beneath areola |
| Correction of Tuberous Nipple Areolar Complex in Gynecomastia | Godwin15 | 2018 | 2 | 13,13 | Secondary correction of tuberous NACs after prior primary glandular reduction. De-epithelialization of upper pole of NAC, superiorly based dermoglandular nipple pedicle. Lower pole excision of skin and areola. | 1 y | Radial scoring of undersurface of NAC if still tuberous |
Our patient exhibited a severe tuberous breast deformity, particularly on the right. In addition to the characteristic features of total base defect and extremely narrow-based breast, a pronounced enlargement of the areolae was also evident well beyond the 2- to 4-cm size typical of male areolae. Options were discussed with the patient and his father, including how to address residual skin redundancy and resize the areolae. Ultimately, pursuit of a free nipple graft (FNG) was chosen. FNG is generally reserved for cases with significant skin excess or recurrent disease.1, 4, 5 FNG was chosen for this patient due not only to the excess skin, but also to achieve more appropriate nipple size, shape, and symmetry in a safe and reliable manner. Though bilateral 7-cm scars will be present with our technique, patients often view these scars as positive marks of distinction and liberation from a debilitating deformity.2
The exact etiology of tuberous breasts remains contested. One theory is that an annular, abnormally constricted investing fascia blocks peripheral expansion of the breast base and promotes areolar herniation. Another theory is that abnormal fascial adherence between the dermis and pectoral muscles impedes peripheral breast bud development.10 Others postulate an attenuated areolar dermal and fascial support system as the etiology, which helps to explain the type 0 tuberous breast.6, 16, 17 Finally, Klinger et al.12 proposed a genetically inherited aberrancy in collagen deposition based on a histologic study of 22 female and five male patients with tuberous breasts. Defining the etiology of the tuberous breast deformity is beyond the scope of this report. Our patient had a family history of tuberous breast deformities in two other male relatives, suggesting a genetic component. Estrogen stimulates breast tissue proliferation, while androgens antagonise estrogen.5 Physiologic imbalance (as might occur in puberty) or pathologic imbalance of these hormones (as might occur in testicular cancer, adrenal tumours, or thyroid disease, among numerous others) can catalyse gynecomastia development. That our patient developed tuberous breasts in the prepubescent period with normal endocrine laboratory findings further supports a genetic, as opposed to anatomic, pathogenesis.
Conclusions
Further research is needed to define the etiology and management of the male tuberous breast. Numerous surgical approaches exist for management. Due to the rarity and uncertainty surrounding this anomaly, careful discussion, thorough examination, and thoughtful surgical planning and execution are essential to achieving positive results.
Disclosures
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for this research, authorship, and publication of this article.
Acknowledgements
The authors would like to acknowledge Melanie Hamilton, RNFA, for her assistance in this work.
References
- 1.Hamilton S., Gault D. The tuberous male breast. Br J Plast Surg. 2003;56(3):295–300. doi: 10.1016/s0007-1226(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 2.Laituri C.A., Garey C.L., Ostlie D.J., St Peter S.D., Gittes G.K., Snyder C.L. Treatment of adolescent gynecomastia. J Pediatr Surg. 2010;45(3):650–654. doi: 10.1016/j.jpedsurg.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Ordaz D.L., Thompson J.K. Gynecomastia and psychological functioning: a review of the literature. Body Image. 2015;15:141–148. doi: 10.1016/j.bodyim.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro D., Horta R., Amarante J., Silva A., Silva P. Tuberous male breast: assessment and esthetic correction. Breast J. 2015;21(6):696–698. doi: 10.1111/tbj.12512. [DOI] [PubMed] [Google Scholar]
- 5.Latham K., Fernandez S., Iteld L., Panthaki Z., Armstrong M.B., Thaller S. Pediatric breast deformity. J Craniofac Surg. 2006;17(3):454–467. doi: 10.1097/00001665-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Costagliola M., Atiyeh B., Rampillon F. Tuberous breast: revised classification and a new hypothesis for its development. Aesthetic plastic surgery. 2013;37(5):896–903. doi: 10.1007/s00266-013-0124-2. [DOI] [PubMed] [Google Scholar]
- 7.Sadove A.M., van Aalst J.A. Congenital and acquired pediatric breast anomalies: a review of 20 years' experience. Plast Reconstr Surg. 2005;115(4):1039–1050. doi: 10.1097/01.prs.0000154214.99641.72. [DOI] [PubMed] [Google Scholar]
- 8.Rees T.D., Aston S.J. The tuberous breast. Clin Plast Surg. 1976;3(2):339–347. [PubMed] [Google Scholar]
- 9.von Heimburg D. Refined version of the tuberous breast classification. Plast Reconstr Surg. 2000;105(6):2269–2270. doi: 10.1097/00006534-200005000-00068. [DOI] [PubMed] [Google Scholar]
- 10.Grolleau J.L., Lanfrey E., Lavigne B., Chavoin J.P., Costagliola M. Breast base anomalies: treatment strategy for tuberous breasts, minor deformities, and asymmetry. Plast Reconstr Surg. 1999;104(7):2040–2048. doi: 10.1097/00006534-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Meara J.G., Kolker A., Bartlett G., Theile R., Mutimer K., Holmes A.D. Tuberous breast deformity: principles and practice. Ann Plast Surg. 2000;45(6):607–611. doi: 10.1097/00000637-200045060-00006. [DOI] [PubMed] [Google Scholar]
- 12.Klinger M., Caviggioli F., Klinger F., Villani F., Arra E., Di Tommaso L. Tuberous breast: morphological study and overview of a borderline entity. Can J Plast Surg. 2011;19(2):42–44. doi: 10.1177/229255031101900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahabedian M.Y. Breast deformities and mastopexy. Plast Reconstr Surg. 2011;127(4):91e–102e. doi: 10.1097/PRS.0b013e31820a7fa7. [DOI] [PubMed] [Google Scholar]
- 14.Haibach H., Rosenholtz M.J. Prepubertal gynecomastia with lobules and acini: a case report and review of the literature. Am J Clin Pathol. 1983;80(2):252–255. doi: 10.1093/ajcp/80.2.252. [DOI] [PubMed] [Google Scholar]
- 15.Godwin Y. Correction of tuberous nipple areolar complex deformity in gynecomastia: the deformity that can get forgotten. Ann Plast Surg. 2018;81(1):3–6. doi: 10.1097/SAP.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 16.Mandrekas A.D., Zambacos G.J. Aesthetic reconstruction of the tuberous breast deformity: a 10-year experience. Aesthetic Surg J. 2010;30(5):680–692. doi: 10.1177/1090820X10383397. [DOI] [PubMed] [Google Scholar]
- 17.Pacifico M.D., Kang N.V. The tuberous breast revisited. J Plast Reconstruct Aesthetic Surg. 2007;60(5):455–464. doi: 10.1016/j.bjps.2007.01.002. [DOI] [PubMed] [Google Scholar]