Abstract
Patient: Male, 43-year-old
Final Diagnosis: ARDS due to Pneumocystis jirovecii pneumonia in AIDS patient
Symptoms: Cough • dyspnea • fever
Medication:—
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Unusual clinical course
Background:
Patients with HIV infection tend to have poor intensive care unit (ICU) outcomes; however, survival in the modern combination antiretroviral therapy (cART) era has markedly improved, but Pneumocystis jirovecii pneumonia (PJP) still remains a preeminent cause of respiratory failure in AIDS patients.
Extracorporeal membrane oxygenation (ECMO) is an adapted cardiopulmonary bypass circuit for temporary life support for patients not responding to conventional treatment.
Case Report:
A 43-year-old male HIV “late presenter” was admitted to our hospital for fever and dyspnea. A chest CT scan revealed bilateral ground-glass opacities. Empiric antibiotic treatment and cART were started. The emergence of ARDS due to PJP dictated urgent veno-venous (VV) ECMO placement. One week later, radiologic findings and respiratory function had improved and the patient was started on a weaning trial from ECMO and removed 12 days after placement.
Conclusions:
Acute respiratory distress syndrome (ARDS) is a potentially reversible clinical syndrome with a high mortality rate. ECMO is a rescue therapy allowing lung recovery during acute processes and should be considered an adequate treatment option in HIV+ patients with respiratory failure. ECMO should be considered a useful and adequate treatment option in AIDS patients who have a high risk of dying from respiratory failure.
MeSH Keywords: AIDS-Related Opportunistic Infections; Extracorporeal Circulation; Pneumocystis jirovecii; Respiratory Distress Syndrome, Adult
Background
Although patients with HIV infection tend to have poor ICU outcomes, survival in the modern highly active cART era has markedly improved, with an estimate ICU survival rate of 60–75% [1].
Pneumocystis jirovecii pneumonia (PJP) is a seriously overwhelming complication of HIV infection, with mortality rates as high as 43% when mechanical ventilation is required and, together with bacterial pneumonia, it is the most recurrent and challenging cause of ARDS in AIDS patients [1,2].
ARDS is a potentially reversible clinical syndrome with mortality rates as high as 30% to 40% [3]. It is clinically defined as respiratory failure within 1 week of a known insult or new/worsening respiratory symptoms, along with bilateral opacities on chest radiograph or ultrasound, not fully explained by effusion, collapse, or nodules [4]. Furthermore, respiratory failure has not been fully explained by cardiac function or volume overload [5].
Management of ARDS is based around the diagnosis and treatment of infections, respiratory support (such as oxygen supplementation and positive pressure ventilation), cautious fluid governance, and general supportive measures such as nutritional supplementation [5].
Despite optimized standard therapies, some patients experience further clinical deterioration. For these subjects, ECMO represents a rescue therapy providing time for lungs to recover from acute processes.
Extracorporeal membrane oxygenation (ECMO) is an adapted cardiopulmonary bypass circuit in which the blood is oxygenated by circulating outside of the body in a membrane oxygenator, for temporary life support for use in selected patients with potentially reversible respiratory and/or cardiac failure not responding to conventional medical management [3,6–10].
There are 2 main methods: VV-ECMO, which operates cannulating a central vein for subsequent oxygenation and CO2 elimination; and venoarterial ECMO (VA-ECMO), which involves a central artery cannulation and supplies hemodynamic and respiratory support [11]. The veno-venous modality has been recently assessed as supportive therapy in severe acute respiratory failure [12,13].
According to current guidelines [14], extracorporeal life support (ECLS) should be considered for use with patients in hypoxic respiratory failure when the mortality risk is at least 50%, and it is indicated when the mortality risk is 80% or higher. Risks and benefits for each patient have to be considered individually [14].
Relative contraindications linked to a poor result in patients with respiratory failure, despite ECLS, are: mechanical ventilation at high levels for 7 days or more, major pharmacologic immunosuppression, recent or expanding CNS hemorrhage, major CNS damage or terminal malignancy, and older age [3,7,14].
Up until now, use of ECMO has been avoided in immunocom-promised patients because it can further suppress the immune system, and no clinical indications for HIV-infected or AIDS patients have been established [15]. Moreover, although there are currently no absolute contraindications to using ECMO in these patients [16], just a few cases of ARDS treated by VV-ECMO have been reported, with variable outcomes, and very few AIDS patients with respiratory failure during PJP are reported to survive after ECMO in developed countries [11,17–23].
Here, we report the successful VV-ECMO treatment of an AIDS patient with ARDS complicating a PJP. We hope that this report, together with the associated literature, will improve the knowledge and management of these conditions.
Case Report
A 43-year-old man was admitted at our hospital because of 7 days of high fever (T max 38.5°C). His recent medical history reported recurring cough not responding to empiric treatments, which deteriorated with dyspnea. He had an unremarkable clinical history. He had never undergone major surgery or medical invasive procedures. As he had no comorbidities, he did not take any drugs. His medical family history was also unremarkable.
At admission, the patient was febrile (peaking up 39°C), blood pressure was 95/40 mmHg, heart rate was 124 bpm, and oxygen saturation was 94% on room air. Blood testing showed leukopenia with lymphocytopenia, and a chest X-ray and high-resolution thorax CT scan evidenced bilateral ground-glass opacities. AIDS was diagnosed. His CD4 cell count was 10 cell/μl and HIV-RNA was 469 654 copies/ml.
Bronchoscopy was not performed; a sputum smear examination was negative for PJP, acid-fast bacilli (AFB) and other bacteria, but it was positive for Candida spp.
Empiric treatment was started with sulfamethoxazole/trimethoprim, caspofungin, azithromycin, rifampicin, and steroids, and antiretroviral treatment with tenofovir/emtricitabine and raltegravir.
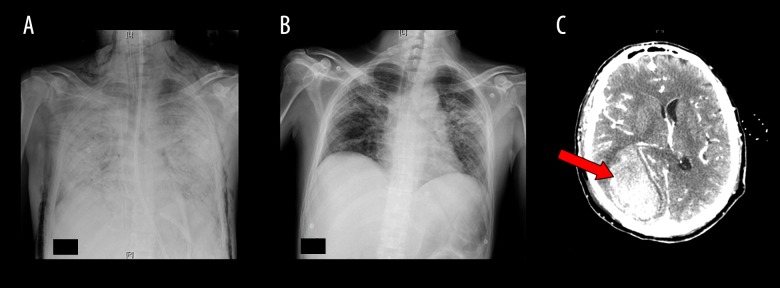
Six days later, after an initial improvement, therapy failed to improve respiratory patterns, and an unexpected ARDS was observed. The patient was then intubated and transferred to the ICU. On day 4 in the ICU, after conventional treatment with optimal mechanical ventilation, a worsened chest X-ray result (Figure 1A), hypoxia (paO2 68 with 100% FiO2), and hyper-capnia (paCO2 68) were sufficiently severe to require urgent VV-ECMO placement before transferring him to the regional ECMO referral Center.
Figure 1.
(A) Chest X-ray before ECMO showing bilateral diffused opacities, (B) improvement of the pulmonary condition after VV-ECMO removal, and (C) CT of the brain at the time of hemorrhage (red arrow) involving temporo-parietal-occipital parenchyma with mass effect.
The patient was started on VV-ECMO support (miniaturized tip-tip-tip heparin-coated circuit; Cardiohelp System; Maquet, Rastatt, Germany). Vessel cannulation [18 Fr return (jugular) and 24 Fr drainage (femoral)] was performed after the administration of a heparin bolus. Blood flow was kept at 4 L/min (about 65–70% of cardiac output) and sweep gas at 4–5 L/min with 100% FiO2. Anticoagulant therapy with unfractionated heparin was also administered. The RESPSCORE was 2.
A second bronchoscopy was performed and PJP was identified, whereas molecular biology (BIOFIRE® FILMARRAY® RP Panel) excluded other pathogens. Comprehensive screening ruled out other opportunistic infections, whereas PCR on blood showed positive Cytomegalovirus (CMV) DNA (1000 copies/mL) and a fundus examination revealed CMV retinitis.
Therapy was de-escalated, relying on sulfamethoxazole/trim-ethoprim, steroids, and caspofungin combination, while cART was confirmed; anti-CMV therapy started with Ganciclovir. After 3 days, his temperature decreased until defervescence, and the hemodynamic picture improved enough to allow nor-adrenaline weaning.
One week later, radiologic findings (Figure 1B) and respiratory function improved and the patient was started on a weaning trial from ECMO, and was removed on day 16 of ICU stay.
On day 18 of ICU stay, the second day after ECMO removal, the patient, still sedated and checked for complications as per protocol, presented abrupt anisocoria. A brain CT scan revealed a hemorrhage involving temporo-parietal-occipital parenchyma with mass effect (Figure 1C). Neurosurgical evacuation was promptly performed, without complications.
On day 34 of ICU stay, the team proceeded with extubation. The patient maintained good respiratory patterns with a mist mask, and no motor or sensory deficits remained. He was switched to secondary prophylaxis with oral sulfamethoxazole/trimethoprim and valganciclovir. On day 37 of ICU stay, he was transferred to the general ward and then admitted to a rehabilitation program. Finally, he was admitted to the HIV out-patient unit when his CD4 cell count was 469 lymphocyte/μl and HIV-RNA 508 copies/ml. At 8 months after the onset of PJP, when his CD4 cell count was 519 cell/μl and HIV-RNA was 103 copies/ml, oral valganciclovir was stopped. The patient was able to resume working and he had a complete recovery.
Discussion
We successfully used ECMO in an AIDS patient with ARDS secondary to PJP associated with an undiagnosed HIV infection. Our patient presented with an extremely high HIV viral load and a very low CD4 count, likely correlating with the extended period of untreated infection, and making him a “late presenter”. In addition, the diagnosis of PJP defined him as an AIDS patient.
While some authors contraindicated ECMO treatment in patients with AIDS [13], other authors reported a non-inferior mortality compared to the general population in patients with well-controlled HIV infection and a satisfactory immune status [24].
An increasing number of AIDS patients with respiratory failure due to PJP pneumonia are reported to be treated with ECMO therapy [17,18,20,21,25,26] and, as with the case described here, most reported cases of PJP treated with ECMO were managed using the VV modality, only 1 receiving with VA-ECMO (Table 1). Moreover, regardless of CD4+ cell count and HIV-RNA load, the majority of patients survived after ECMO treatment, as did the case reported here.
Table 1.
Adult HIV/AIDS patients with severe Pneumocystis jirovecii pneumonia necessitating ECMO therapy (literature review).
| Patient (Ref) | Age (years)/Sex (M/F) | ECMO configuration | Duration of ECMO (days) | CD4 count (cells/mm3) | HIV viral load (copies/mL) | Outcome |
|---|---|---|---|---|---|---|
| Gutermann et al. [18] | 55/M | Veno-arterial | 4 | 9 | 80.235 | Survived |
| Steppan et al. [27] | 39/M | Veno-venous | 14 | 69 | 6297 | Died on ECMO |
| Goodman et al. [20] | 25/M | Veno-venous | 69 | 36 | 622.234 | Died on ECMO |
| Goodman et al. [20] | 30/F | Veno-venous | 7 | 13 | 976.631 | Survived |
| De Rosa et al. [21] | 21/F | Veno-venous | 20 | 2 | 118.330 | Survived |
| De Rosa et al. [21] | 24/M | Veno-venous | 24 | 3 | 50.728 | Died post ECMO |
| Cawcutt et al. [17] | 45/M | Veno-venous | 57 | 33 | 113.000 | Died post ECMO |
| Husain et al. [25] | 26/M | Veno-venous | 6 | 84 | 907.302 | Survived |
| Stahl et al. [28] [Case series (6 cases)] | Median age 41 yo/75% Male | Veno-venous | – | – | – | – |
| Obata et al. [29] | 47/M | Veno-venous | 19 | 6 | 150.000 | Survived |
| Simpson et al. [1] | 35/M | Veno-venous | 27 | – | 1.269.866 | Survived |
| Horkita et al. [26] | 23/M | Veno-venous | 26 | 8 | 550.000 | Survived |
| Hernandez et al. [16] | 29/M | Veno-venous | 19 | 4 | 131.000 | Survived |
| Morley et al. [22] | 33/M | Veno-venous | 21 | 133 | 83.000 | Survived |
| Capatos et al. [30][Case series (15 cases)] | Median age 39 yo/5% Male | Veno-venous | (Median duration) 12 | (Median count) 19.5 | 190.574 (median Viral load) | 60% survived |
In addition, although our patient was older than the others listed in Table 1, he had no comorbidities and he was promptly started on ECMO treatment. These facts, along with the appropriate treatment of PJP and HIV infection, led to the successful result.
Thus, as in other reports, our case suggests that ECMO is an option to consider and is a practical rescue modality for the treatment of AIDS patients with ARDS due to PJP, if promptly initiated during their hypoxemic respiratory failure and associated with appropriate support therapies.
In this scenario, it is also crucial to rule out other respiratory infections, whether opportunistic or not, such as tuberculosis, non-tubercular mycobacterial infections, Legionellosis, Mycoplasma pneumonia, and viral pneumonia. After the PJP diagnosis, we were able to exclude other pathogens using molecular biology in bronchoscopy samples.
Finally, because of our patient’s very low CD4+ cell count (10 cell/μl) and the development of PJ pneumonia, we could not exclude that an immune reconstitution inflammatory syndrome (IRIS) played a role to the ARDS presentation, along with CMV retinitis, although this is only a clinical speculation.
Conclusions
Our findings confirm that ECMO support may be justified in immunocompromised patients with PJP-associated ARDS with a high risk of dying from respiratory failure. This is one of the few case reports of AIDS patients surviving after decannulation from ECMO and successfully discharged from the hospital. It should be useful to speculate on the baseline characteristics of patients to identify predictive criteria of positive outcome. Moreover, this case report is unique not only for the discussion of clinical management, but also because the patient was managed in 5 different clinical units, achieving prompt management of his clinical situation.
Acknowledgments
We thank Prof. Stefano Rusconi of University of Milan for revision of the article. We thank Dr. Pietro Leanza for English-language revision.
Footnotes
Conflict of Interest
None.
References:
- 1.Collett LW, Simpson T, Camporota L, et al. The use of extracorporeal membrane oxygenation in HIV-positive patients with severe respiratory failure: A retrospective observational case series. Int J STD AIDS. 2019;30(4):316–22. doi: 10.1177/0956462418805606. [DOI] [PubMed] [Google Scholar]
- 2.Marino A, Caltabiano E, Zagami A, et al. Rapid emergence of cryptococcal fungemia, Mycobacterium chelonae vertebral osteomyelitis and gastro intestinal stromal tumor in a young HIV late presenter: A case report. BMC Infect Dis. 2018;18(1):693. doi: 10.1186/s12879-018-3573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuerer DJE, Kolovos NS, Boyd KV, Coopersmith CM. Extracorporeal membrane oxygenation. Chest. 2008;134(1):179–84. doi: 10.1378/chest.07-2512. [DOI] [PubMed] [Google Scholar]
- 4.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–96. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 7.Mount DB. Collectrin and the kidney. Curr Opin Nephrol Hypertens. 2007;16(5):427–29. doi: 10.1097/MNH.0b013e3282e9acc5. [DOI] [PubMed] [Google Scholar]
- 8.Gray B, Rintoul N. ELSO neonatal respiratory failure supplement to the ELSO general guidelines Extracorporeal Life Support Organization (ELSO) guidelines for neonatal respiratory failure. ELSO Guidel Adult Respir Fail Guidel. 2017 Dec;:1–34. [Google Scholar]
- 9.Sidebotham D. Extracorporeal membrane oxygenation – understanding the evidence: CESAR and beyond. J Extra Corpor Technol. 2011;43(1):P23–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest P, Ratchford J, Burns B, et al. Retrieval of critically ill adults using extracorporeal membrane oxygenation: An Australian experience. Intensive Care Med. 2011;37(5):824–30. doi: 10.1007/s00134-011-2158-8. [DOI] [PubMed] [Google Scholar]
- 11.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365(20):1905–14. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 12.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–75. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 13.Davies AR, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 14.Extracorporeal Life Support Organization (ELSO) ELSO Guidelines for Adult Respiratory Failure v1.4. Extracorpor Life Support Organ. 2017 Aug;:1–32. [Google Scholar]
- 15.Rihal CS, Naidu SS, Givertz MM, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino America. J Am Coll Cardiol. 2015;65(19):e7–26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez Conte AT, Ng D, Ramzy D, et al. Extracorporeal membrane oxygenation in a 29-year-old man with Pneumocystis jirovecii respiratory failure and AIDS. Texas Hear Inst J. 2018;45(4):254–59. doi: 10.14503/THIJ-16-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cawcutt K, Gallo De Moraes A, et al. The use of ecmo in hiv/aids with Pneumocystis jirovecii pneumonia: A case report and review of the literature. ASAIO J. 2014;60(5):606–8. doi: 10.1097/MAT.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 18.Gutermann H, Van Roy B, Meersseman W, et al. Successful extracorporeal lung assistance for overwhelming pneumonia in a patient with undiag-nosed full blown AIDS – A controversial therapy in HIV-patients. Thorac Cardiovasc Surg. 2005;53(4):252–54. doi: 10.1055/s-2005-837644. [DOI] [PubMed] [Google Scholar]
- 19.Park M, Azevedo LCP, Mendes PV, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics. 2012;67(10):1157–63. doi: 10.6061/clinics/2012(10)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman JJ, Goodman LF, Sarvepalli SK, et al. Extracorporeal membrane oxygenation as adjunctive therapy for refractory hypoxemic respiratory failure in HIV-positive patients with severe Pneumocystis jirovecii pneumonia. Clin Pulm Med. 2013;20(3):117–20. [Google Scholar]
- 21.De Rosa FG, Fanelli V, Corcione S, et al. Extra Corporeal Membrane Oxygenation (ECMO) in three HIV-positive patients with acute respiratory distress syndrome. BMC Anesthesiol. 2014;14:37. doi: 10.1186/1471-2253-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley D, Lynam A, Carton E, et al. Extracorporeal membrane oxygenation in an HIV-positive man with severe acute respiratory distress syndrome secondary to pneumocystis and cytomegalovirus pneumonia. Int J STD AIDS. 2018;29(2):198–202. doi: 10.1177/0956462417725447. [DOI] [PubMed] [Google Scholar]
- 23.Capatos G. ECMO in the HIV population. Qatar Med J. 2017;2017(1):45. [Google Scholar]
- 24.Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS. 2013;27(6):973–79. doi: 10.1097/QAD.0b013e32835cae9c. [DOI] [PubMed] [Google Scholar]
- 25.Ali HS, Hassan IF, George S. Extra corporeal membrane oxygenation to facilitate lung protective ventilation and prevent ventilator-induced lung injury in severe Pneumocystis pneumonia with pneumomediastinum: A case report and short literature review. BMC Pulm Med. 2016;16(1):52. doi: 10.1186/s12890-016-0214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horikita S, Sanui M, Fujimoto Y, Lefor AK. Successful repeat ECMO in a patient with AIDS and ARDS. BMJ Case Rep. 2017;2017:bcr2017219870. doi: 10.1136/bcr-2017-219870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steppan J, Sikazwe I. Extra-corporeal membrane oxygenation in an adult with severe Pneumocystis pneumonia [Abstract] Baltimore: American College of Physicians Meeting; 2009. [Google Scholar]
- 28.Stahl K, Schenk H, Seeliger B, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome due to Pneumocystis pneumonia. Eur Respir J. 2019;54(3):1900410. doi: 10.1183/13993003.00410-2019. [DOI] [PubMed] [Google Scholar]
- 29.Obata R, Azuma K, Nakamura I, Oda J. Severe acute respiratory distress syndrome in a patient with AIDS successfully treated with veno-venous extracorporeal membrane oxygenation: A case report and literature review. Acute Med Surg. 2018;5(4):384–89. doi: 10.1002/ams2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capatos G, Burke CR, Ogino MT, et al. Venovenous extracorporeal life support in patients with HIV infection and Pneumocystis jirovecii pneumonia. Perfus (United Kingdom) 2018;33(6):433–37. doi: 10.1177/0267659118765595. [DOI] [PubMed] [Google Scholar]