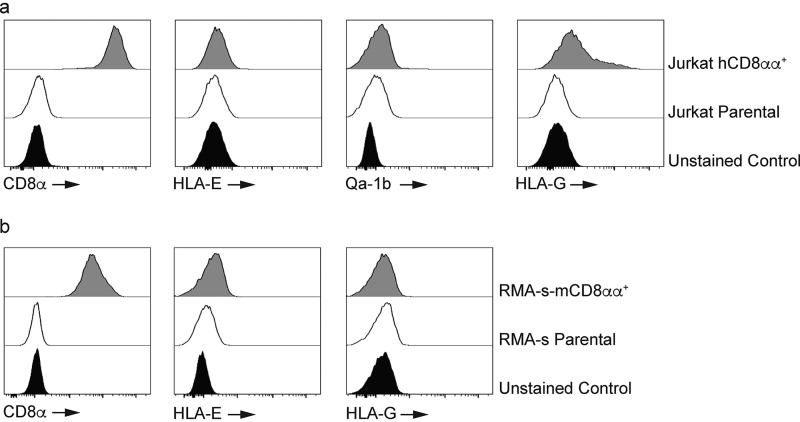
Figure 4.
Qa-1b and HLA-E differ in their ability to bind CD8αα. a, Jurkat cells were engineered to express human CD8αα (hCD8αα). Antibody staining shows a population of Jurkat-CD8αα cells with high expression of the CD8α homodimer (left panel). The filled histogram is the unstained control whereas the open histogram is CD8 staining on Jurkat cells. The shaded histogram is CD8 staining on Jurkat-CD8αα cells. Results are representative of at least two independent experiments. All histograms have been offset to stack vertically above one another and scaled to maximum count for clarity. Tetramer staining shows that HLA-E and Qa-1b (second and third panels) have minimal binding to human CD8αα whereas HLA-G (right panel) interacts with human CD8αα. The filled histograms are the unstained controls whereas the open histograms are tetramer staining on Jurkat cells. The shaded histograms are tetramer staining on Jurkat-CD8αα cells. Results are representative of at least two independent experiments. All histograms have been offset to stack vertically above one another and scaled to maximum count for clarity. b, HLA-E and HLA-G show minimal interaction with mouse CD8αα (mCD8αα). Antibody staining of RMA-s-mCD8αα cells (left panel). The filled histogram is the unstained control whereas the open histogram is CD8 staining on RMA-s cells. The shaded histogram is CD8 staining on RMA-s–mCD8αα cells. Results are representative of at least two independent experiments. All histograms have been offset to stack vertically above one another and scaled to maximum count for clarity. Tetramer staining shows that HLA-E (middle panel) and HLA-G (right panel) do not bind mouse CD8αα. The filled histograms are the unstained controls whereas the open histograms are tetramer staining on RMA-s cells. The shaded histograms are tetramer staining on RMA-s–mCD8αα cells. Results are representative of at least three independent experiments. All histograms have been offset to stack vertically above one another and scaled to maximum count for clarity.