Figure 4.
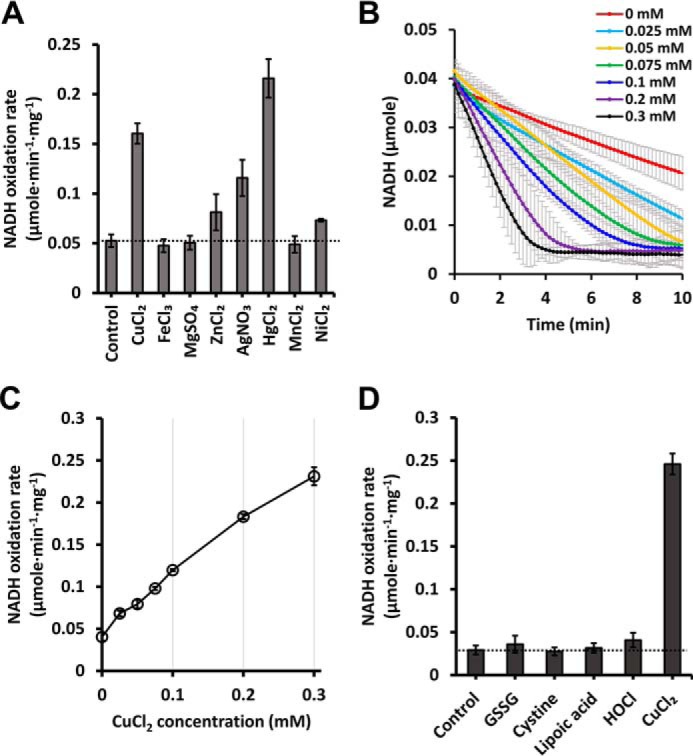
NADH oxidation of EcRclA in the presence of various substrates. A, NADH oxidation rates depend on various transition metal ions. NADH oxidation rates by RclA were measured over time spectrophotometrically (n = 3, mean ± S.D.). The reaction was initiated by injecting 200 μm NADH into a buffer containing 2 μm RclA with or without 100 μm of metal ions. A control experiment was performed without metal ion. The horizontal line indicates the reference NADH oxidation rate of control. B, NADH oxidation profiles with different concentrations of Cu2+. The reaction was carried out in a buffer containing 200 μm NADH and indicated concentrations of CuCl2 (from 0 to 0.3 mm). The amounts of the NADH oxidation were measured spectrophotometrically (n = 3, mean ± S.D.). C, initial NADH oxidation rates calculated from the NADH oxidation profile in Fig. 4B. The reaction was assayed with 200 μm NADH and marked concentrations of Cu2+. The NADH oxidation rates were measured over time spectrophotometrically (n = 3, mean ± S.D.). D, NADH oxidation rates with various substances. The reaction was initiated by injecting 200 μm NADH into a buffer containing 2 μm RclA and 200 μm of various materials. A control experiment was performed without material. NADH oxidation rates were measured over time spectrophotometrically (n = 3, mean ± S.D.)
