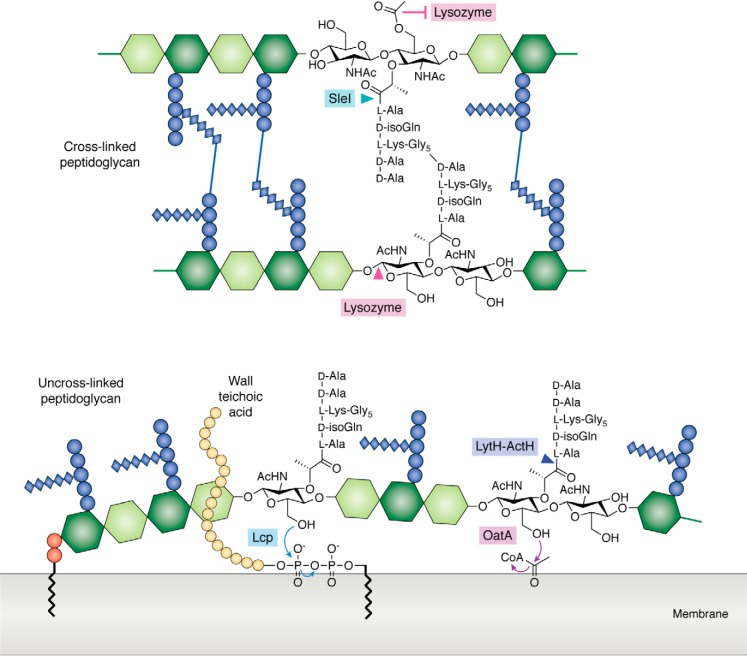
Figure 6.
Peptidoglycan hydrolases and tailoring enzymes have distinct substrate preferences. In S. aureus, the LCP wall teichoic acid ligases and the LytH-ActH amidase-activator complex are membrane-anchored proteins that only act on uncross-linked peptidoglycan substrates. The S. aureus O-acetyltransferase OatA is also a membrane-anchored protein and may act preferentially on uncross-linked peptidoglycan. By contrast, S. aureus Sle1 is an amidase that acts on cross-linked peptidoglycan. Another example of substrate selectivity is provided by lysozyme, which cleaves unacetylated, but not O-acetylated, backbones. Substrate selectivity can offer insights into the functions of these enzymes, as those enzymes that act at an earlier stage of peptidoglycan synthesis may show a preference for nascent peptidoglycan substrates.