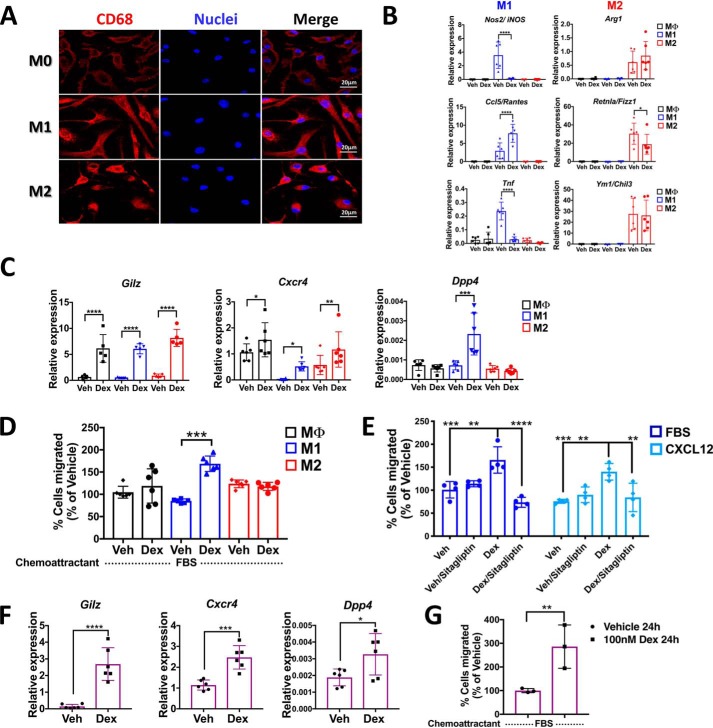
Figure 6.
Glucocorticoids up-regulate Dpp4 expression in mouse BMDMs polarized to classically activated profile M1 and peritoneal macrophages promoting migration. BMM isolated by negative selection and differentiated into BMDM with 100 μg/ml M-CSF were evaluated by immunofluorescence microscopy. A, immunofluorescence staining of in vitro cultured mouse M0, M1, and M2 macrophages labeled with the macrophage marker CD68. Scale bar, 20 μm. B, gene expression profiles by qRT-PCR of M1 (Nos2, Ccl5, and Tnf) and M2 (Arg1, Retnla, and Ym1) markers at 6 h with 100 nm Dex. C, mRNA levels of Gilz, Cxcr4, and Dpp4 regulated by glucocorticoids in M0, M1, and M2 macrophages. D, in vitro migration assay, using 10% FBS as chemoattractant, of M0, M1, and M2 macrophages treated with or without dexamethasone. After 24 h, M1 macrophages show a significant increase in spontaneous migration upon Dex treatment. E, the cell migration of primary M1 macrophages induced by CXCL12 is blocked by pharmacological inhibition of DPP4 following dexamethasone treatment. F, mRNA levels of Gilz, Cxcr4, and Dpp4 regulated by glucocorticoids in mouse PM. G, in vitro migration assay, using 10% FBS as chemoattractant, of PM treated with or without dexamethasone shows an increase in the migratory potential induced by glucocorticoids. Data are mean ± S.D. (error bars) and are representative of 3–5 independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ordinary one-way ANOVA statistical test with Tukey's multiple-comparison test and unpaired two-tailed t test.