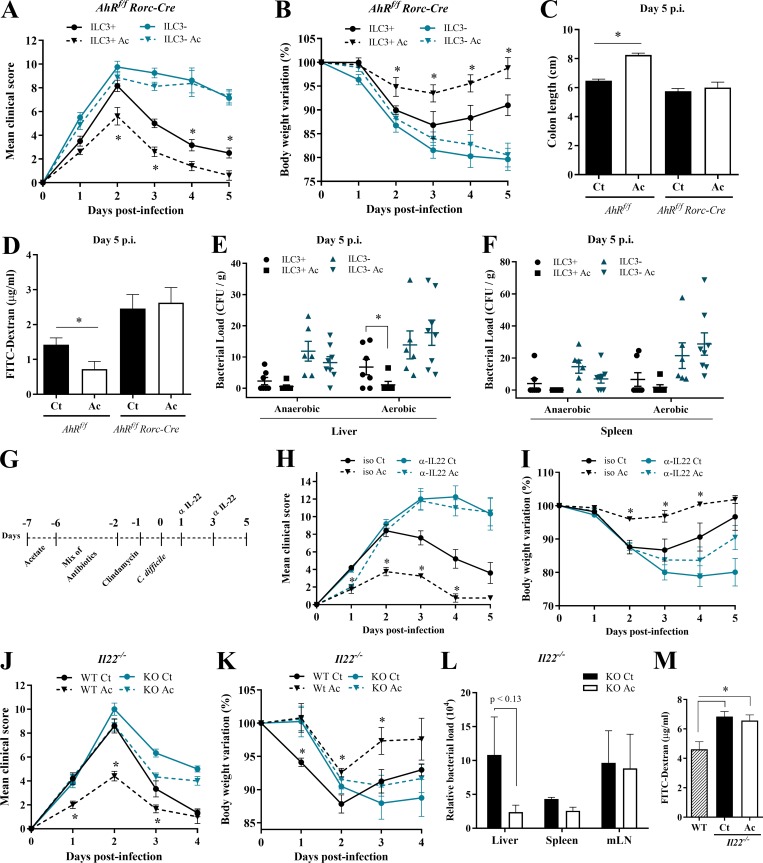
Figure 6.
IL-22–producing ILC3s are essential for acetate-mediated protection during CDI. (A–D) Clinical score (A), body weight changes (B), colon length (C), and intestinal permeability (D) in ILC3-sufficient (AhRfl/fl) and -deficient (AhRfl/fl × Rorc-Cre) mice that were treated (Ac) or not (Ct) with acetate and infected with C. difficile (n = 5–8). AhRfl/fl × Rorc-Cre and AhRfl/fl littermates were infected at the same time. (E and F) Bacterial translocation into the liver (E) and spleen (F) in ILC3-sufficient and -deficient mice treated or not with acetate and infected with C. difficile. Samples were collected 5 d p.i., plated in blood agar, and incubated for 4 d at 37°C in aerobic or anaerobic conditions (n = 5–8). (G) Infected mice were treated with or without acetate and received an i.p. dose of anti–IL-22 neutralizing antibody or isotype (iso) control IgG2a on days 1 and 3 p.i. (H and I) Mice were monitored for clinical score (H) and weight change (I) until day 5 p.i. (n = 5). (J and K) Clinical score (J) and weight changes (K) of IL22−/− and WT mice infected with 108 CFU of C. difficile that were either treated or not with acetate (n = 5–6). IL22−/− and WT mice were bred in the same animal facility, matched for sex and age, and infected at the same time to avoid batch effects. (L) Bacterial translocation into peripheral organs on day 2 p.i. in IL22−/− mice treated or not with acetate (n = 5–6). (M) Intestinal permeability of IL22−/− mice treated or not with acetate and infected with C. difficile (n = 5–6). Infected WT mice not treated with acetate are shown as controls. Results are representative of at least two independent experiments with four to six mice in each experimental group (G–I) or pooled results from two independent experiments with three to four mice in each group (A–F). Results are presented as mean ± SEM. *, P < 0.05.