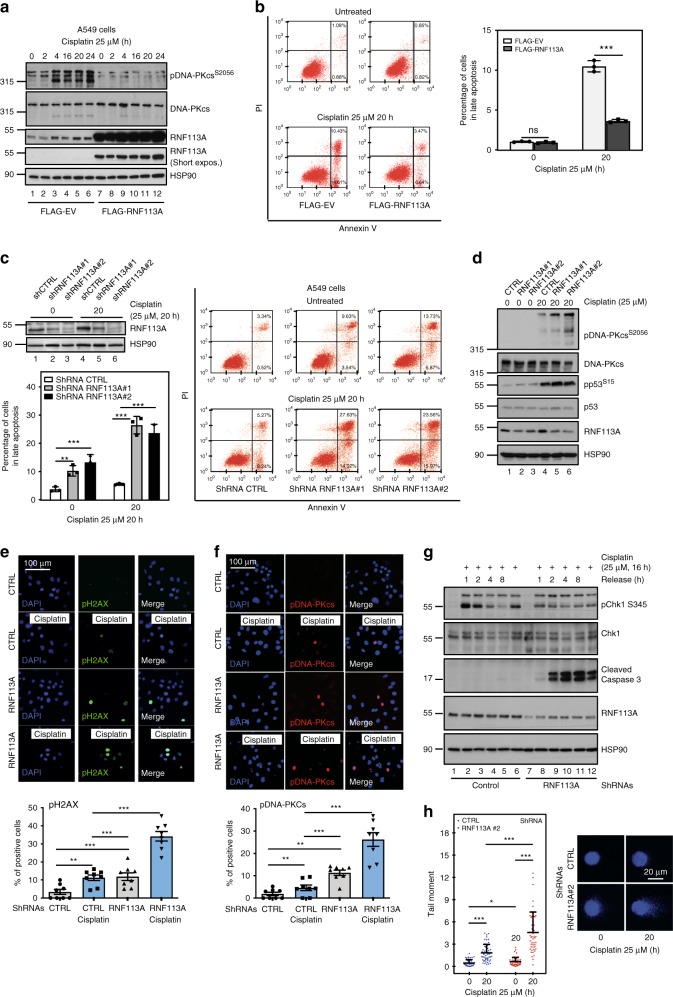
Fig. 2. RNF113A limits Cisplatin-dependent cell death.
a RNF113A overexpression interferes with DNA-PKcs phosphorylation upon Cisplatin treatment. Control or RNF113A-overexpressing A549 cells were stimulated or not with Cisplatin and WB analyses were done. b RNF113A overexpression limits Cisplatin-dependent cell death. Control or RNF113A-overexpressing A549 cells were untreated or stimulated with Cisplatin. The percentage of cells in early (Annexin V positive and PI negative) or late apoptosis (Annexin V positive and PI positive) was assessed by FACS. On the left, FACS data from one representative experiment. On the right, the histogram from two independent experiments (Student t-test, p-values: ***<0.001). ns = non significant. c RNF113A deficiency enhances Cisplatin-mediated cell death. Extracts from control (“ShCTRL”) or from RNF113A-depleted (“ShRNF113A#1 and ShRNF113A#2”) A549 cells were subjected to WB analyses. Cell survival upon Cisplatin treatment was assessed by FACS (right panel). On the left, FACS data from two independent experiments are illustrated in the histogram (Student t-test, ***p < 0.001). d RNF113A negatively regulates Cisplatin-induced DNA-PKcs phosphorylation. Control or RNF113A-depleted BZR-T33 cells were untreated or stimulated with Cisplatin and WB analyses were done. e, f. RNF113A deficiency enhances the number of pH2AX (S139) (e) and pDNA-PKcs (S2056)+ (f) cells upon Cisplatin treatment. Control and RNF113A-deficient A549 cells were treated with Cisplatin (25 μM) for 4 h and immunofluorescence analyses were done to quantify the number of pH2AX+ or pDNA-PKcs (S2056)+ cells. The corresponding histogram represents 10 blindly taken fields containing at least 150 nuclei per field (Student t-test, ***p < 0.001, **p < 0.01). g RNF113A deficiency impairs ATR activation upon DNA damage. Control or RNF113A-depleted A549 cells were treated or not with Cisplatin and then allowed to grow in a fresh media for the indicated periods of time. Chk1 phosphorylation was assessed by WB. h RNF113A promotes DNA repair. Control versus RNF113A-depleted BZR-T33 cells were treated with Cisplatin and an alkaline Comet Assay was done. Fifty images per conditions were analyzed by OpenComet in ImageJ. The tail moment of every cell was calculated in control versus RNF113A-depleted BZR-T33 cells. Data are shown as mean ± SD (***p < 0.001, *p < 0.05, Student t-test).