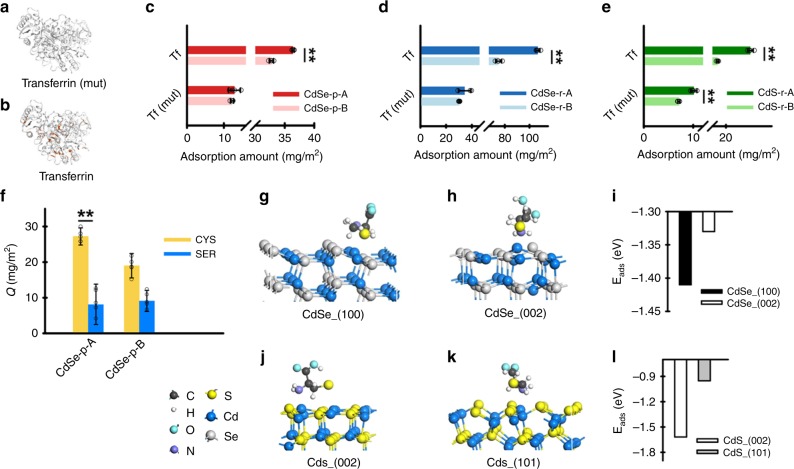
Fig. 4. Facet-dependent preferential binding of transferrin with Cd nanocrystals occurred through thiol complexation.
a, b Schematic description of the structures of transferrin and non-thiol transferrin (mut) (cysteine shown in red). Adsorption of transferrin on CdSe nanoparticles (c, CdSe-p-A: dark red bar; CdSe-p-B: light red bar; p = 0.0006 for transferrin and 0.4071 for transferrin (mut)), CdSe nanorods (d, CdSe-r-A: dark blue bar; CdSe-r-B: light blue bar; p < 0.0001 for transferrin and =0.1306 for transferrin (mut)) and CdS nanorods (e, CdS-r-A: dark green bar; CdS-r-B: light green bar; p = 0.0003 for transferrin and 0.0006 for transferrin (mut)) was consistently greater compared with the non-thiol transferrin (mut). Data are presented as mean ± SD of four replicate samples, and analyzed by one-way ANOVA (n = 4). Competitive adsorption of cysteine (CYS) vs. serine (SER) (f, CYS: yellow bar; SER: blue bar) on the two different-faceted CdSe nanoparticles showed that thiol-containing compounds preferentially adsorbed to the “A” material. Data are presented as mean ± SD of five replicate samples (n = 5; p = 0.0098 for cysteine and 0.8567 for serine by one-way ANOVA). Density function theory (DFT) calculation indicated that cysteine exhibited lower adsorption energy toward (100) vs. (002) of CdSe (g–i, CdSe_(100): black bar; CdSe_(002): white bar), and exhibited lower adsorption energy toward (002) vs. (101) of CdS (j–l, CdS_(002): white bar; CdS_(101): gray bar). Statistical significance between groups: **p < 0.01. Source data are provided as a Source Data file.