Abstract
Abnormal functional connectivity (FC) within discrete brain networks is involved in the pathophysiology of obsessive-compulsive disorder (OCD) with inconsistent results. In the present study, we investigated the FC patterns of 40 drug-naive patients with OCD and 38 healthy controls (HCs) through an unbiased voxel-wise global brain FC (GFC) analysis at rest. Compared with HCs, patients with OCD showed decreased GFC within the default mode network (DMN) (i.e., left posterior cingulate cortex/lingual gyrus) and sensorimotor network (i.e., left precentral gyrus/postcentral gyrus) and increased GFC within the executive control network (ECN) (i.e., left dorsal lateral prefrontal cortex and left inferior parietal lobule). Receiver operating characteristic curve analyses further indicated that the altered GFC values within the DMN, ECN, and sensorimotor network may be used as neuroimaging markers to differentiate patients with OCD from HCs. These findings indicated the aberrant FC patterns of the DMN, ECN, and sensorimotor network associated with the pathophysiology of OCD and provided new insights into the changes in brain organization function in OCD.
Keywords: obsessive-compulsive disorder, global brain functional connectivity, functional magnetic resonance imaging, network, resting state
Introduction
Obsessive-compulsive disorder (OCD) is a common and chronic psychiatric disorder involving intrusive, unwanted thoughts, and/or repetitive behavior, anxiety, and social dysfunction. OCD has a lifetime prevalence of 2%–3% worldwide (1), but the underlying neurobiological mechanisms remain unclear.
Neuroimaging studies have shown that OCD may be caused by distortions within large-scale brain networks rather than from independent brain regions (2). A “triple network” model emphasizes the abnormal intrinsic functional connectivity (FC) patterns within and between the executive control network (ECN), salience network (SN), and default mode network (DMN) as essential features of psychiatric disorders including OCD (3). DMN is composed of posterior cingulate cortex (PCC), medial prefrontal cortex (PFC), and lateral posterior cortices, which is involved in reflective and introspective self-awareness processes (4). ECN is composed of dorsal lateral PFC (DLPFC) and posterior parietal cortex, which is crucial for cognitive control (such as planning and response inhibition) (5, 6). SN is composed of anterior insular cortices and dorsal anterior cingulate, which plays an important role in regulating external and internal salient information (3, 7). The DMN deactivates during cognitive demanding tasks and shows increased activities during resting state, and is considered as a task-negative network. The ECN shows elevated co-activation during the cognitive performance, and is a task-positive network. The SN is involved in monitoring the interactions between the DMN and ECN (8). FC within and between the DMN, ECN, and SN was reported to be altered in patients with OCD, and dysfunction of the triple networks may be related to obsessive thoughts and/or compulsive behavior in OCD (2).
Considerable neuroimaging studies have observed altered FC and/or regional activity in brain regions within the DMN, ECN, and SN with inconsistent results. Increased FC and/or regional homogeneity within the DMN, ECN, and SN at rest were found through using the independent component analysis and regions of interest (ROI) seed-based FC approaches (8–12). By contrast, neuroimaging studies have reported decreased FC within the DMN, ECN, and SN at rest in OCD (2, 13–15). Two main factors may explain the inconsistency of the findings. One factor may be the sample heterogeneity regarding medication use. Selective serotonin reuptake inhibitors (SSRIs) may modulate the neural condition and are known to have a remarkable influence on FC at rest in patients with OCD (16, 17). Therefore, drug-naive patients with OCD should be employed to obtain primitive FC information of the neurobiological mechanisms in OCD. Another important factor may be that numerous neuroimaging studies have concentrated on FC between brain regions of an appointed network rather than using a whole brain assessment (18). Thus, FC based on the assumptive network of interest may lead to biased results and limits the investigation of the most remarkably altered brain areas that may represent the essential abnormality in the neurobiological mechanisms of OCD (19). Voxel-wise global brain FC (GFC) analysis is a model-free method used to investigate functional interactions across the whole brain regions with an unbiased hypothesis-driven manner, and has been applied to obtain whole brain FC in patients with major depressive disorder (19), schizophrenia (20), and somatization disorder (21).
In this study, we compared GFC differences between drug-naive patients with OCD and healthy controls (HCs) using resting-state fMRI. On the basis of the aforementioned studies, we hypothesized that patients with OCD would show altered GFC in some brain regions, peculiarly in brain regions of the DMN, ECN, and SN. We also hypothesized that these alterations would be related to clinical variables in OCD.
Materials and Methods
Participants
Forty drug-naive patients with OCD were recruited from the Fourth Affiliated Hospital of Qiqihar Medical University and Qiqihar Mental Health Center. Patients with OCD were diagnosed using the Structured Clinical Interview for DMS-IV (SCID), patient version. Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), 17-item Hamilton Rating Scale for Depression (HAMD), and Hamilton Anxiety Rating Scale (HAMA) were used to assess the severity of OCD, depression symptoms, and anxiety symptoms, respectively. Patients with OCD should have a score of higher than 16 on Y-BOCS and score of less than 18 on HAMD. In current OCD sample, 4 patients were with obsessions, 4 patients were with compulsion, and 32 patients were with both obsessions and compulsion. Forty HCs were recruited from the community and screened using the SCID, nonpatient version. The participants shared the following exclusion criteria: (1) diagnosis of other Axis I and II disorders; (2) a history of major physical diseases and neurological disorders; and (3) nicotine/caffeine dependence or substance/alcohol use disorder. Moreover, HCs with a family history of major psychiatric disorders were excluded. All participants were right-handed and aged from 18 to 60 years old.
This study was approved by the Research Ethics Committee at Qiqihar Medical University, China. All participants were informed regarding the procedures, and signed an informed consent.
Image Acquisition and Preprocessing
Images were acquired by using a 3.0-Tesla GE 750 Signa-HDX scanner (General Electric Healthcare, Waukesha, WI, USA) at the Third Affiliated Hospital of Qiqihar Medical University, Heilongjiang, China. The subjects were instructed to relax, remain motionless (especially the head), keep eyes closed, and stay awake. An echo-planar imaging (EPI) sequence was used to acquire resting-state functional magnetic resonance imaging (RS-fMRI). The parameters were as follows: 33 axial slices, TR = 2,000 ms, TE = 30 ms, FA = 90°, thickness/gap = 3.5 mm/0.6 mm, FOV = 200 × 200 mm, and in-plane resolution = 64 × 64. A total of 240 volumes were collected for 8 min.
The images were manually check to ensure good coverage for all voxels included in a whole-braingray matter mask. Data Processing & Analysis for Brain Imaging (DPABI) software was used to preprocess the imaging data (22). After signal stabilization, slice timing determination and head-motion correction were conducted. The subjects included in the analysis should have a maximum displacement ≤ 2 mm in x, y, or z direction and an angular rotation < 2° on each axis. Two HCs were excluded from further analysis because of excessive head motion. The functional images were normalized to the standard EPI template in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and spatially resampled to a voxel size of 3 mm × 3 mm × 3 mm. The processed images were smoothed with a Gaussian kernel of 4 mm full width at the half maximum. The signal was linearly detrended and band-pass filtered (0.01–0.08 Hz) to reduce high-frequency physiological noise and low-frequency drift. The 24 head motion parameters obtained by rigid body correction, white matter, and cerebrospinal fluid time courses were removed from the images by linear regression. The global signal was included because removing it in the preprocessed RS-fMRI FC data remains debatable. Resting-state fMRI is highly susceptible to head motion and that simply regressing out the effects of the 24 head motion parameters is not sufficient to deal with this issue (23, 24). The analyses were therefore using scrubbing with a framewise displacement (FD) measure, which indexes volume-to-volume changes in head position using a threshold of 0.2 together with one preceding and two subsequent volumes (25).
Global-Brain Functional Connectivity (GFC) Analysis
Voxel-wise GFC was calculated in MATLAB within a gray matter mask, which was created by thresholding (probability > 0.2) the gray matter probability map in SPM8 (26). GFC values for a given voxel of each participant were computed between this voxel and all other voxels within the gray matter mask. The formula used to calculate the GFC values was described by Cui et al. (19) as follows:
In this formula, the symbols a and b stand for the given voxels a and b, n represents number, r means the Pearson' s correlation coefficient, Ta and Tb are the time series of voxel a and voxel b. The coefficient r was computed and transformed to z values with Fisher r-to-z transformation (27). GFC of a voxel was defined as the mean coefficient of the given voxel with all other voxels, and GFC maps were created by combining the GFC of all voxels.
Statistical Analysis
Imaging data and demographics were compared between OCD and HCs. Two-sample t-tests were used to analyze the continuous variables, and chi-square tests were used to analyze the categorical data.
GFC analysis was conducted with two-sample t-tests between patients with OCD and HCs. The FD values of each participant were calculated based on the study of Power et al. (25) by indexing volume-to-volume changes in head position. The mean FD values and age were used as covariates to minimize the potential effects of these variables. The significance level was set at the corrected p < 0.05 for multiple comparisons using the Gaussian Random Field (GRF) method (voxel significance: p < 0.001, cluster significance: p < 0.05).
Pearson and Spearman correlation analyses were used to assess the relationship between the GFC values showing significant group differences (mean z values of GFC were extracted) and clinical variables in the patients with OCD. A partial correlation analysis was also calculated between the altered GFC values and clinical variables in OCD, the mean FD values and age were used as covariates. The significance level was Bonferroni corrected at p < 0.05.
Moreover, receiver-operating characteristic curve (ROC) analysis was conducted to determine whether the altered GFC values of these ROIs could be used to differentiate patients with OCD from HCs. A “leave-one-out” method was applied to conduct the ROC. Furthermore, a permutation test was used to validate the ROC results, which ran 10,000 times for each sample (OCD/HCs) and a global accuracy could be obtained for each sample.
Results
Characteristics of the Participants
Supplementary Table S1 indicates the clinical characteristics of all participants. The patients had a mean age of 27.28 ± 8.16 years, a mean duration of illness of 66.68 ± 75.54 months, and a mean education of 13.40 ± 2.87 years. The controls had a mean age of 27.18 ± 8.33 years and a mean education of 13.74 ± 3.03 years. No significant difference was found regarding gender (x2 = 0.32, p = 1.00), age (t = 0.05, p = 0.71), education (t = -0.50, p = 0.83), and FD values (t = 1.25, p = 0.13) between OCD and HC groups. However, significant group differences were found in Y-BOCS total (t = 25.27, p < 0.001) and subscale scores (obsessive thinking, t = 17.98, p < 0.001; compulsive behavior, t = 14.92, p < 0.001), HAMD scores (t = 9.04, p < 0.001), and HAMA scores (t = 9.00, p < 0.001) ( Supplementary Table S1 ).
Group Differences in GFC
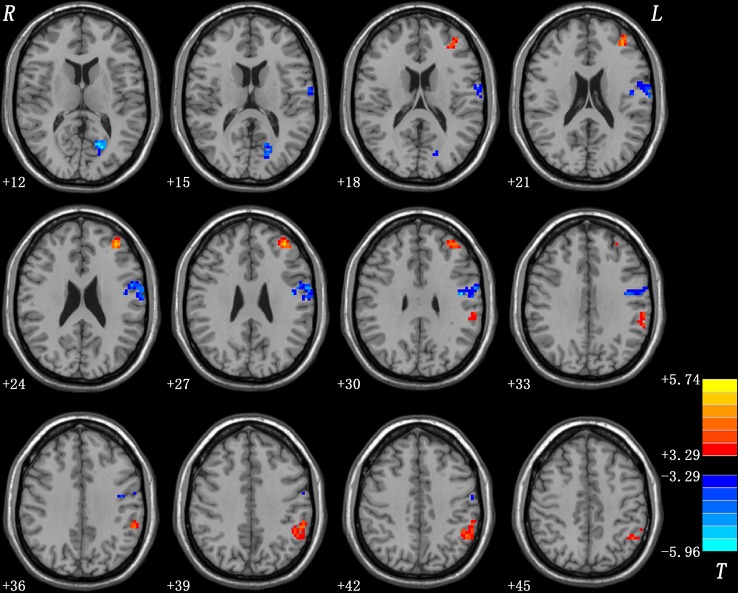
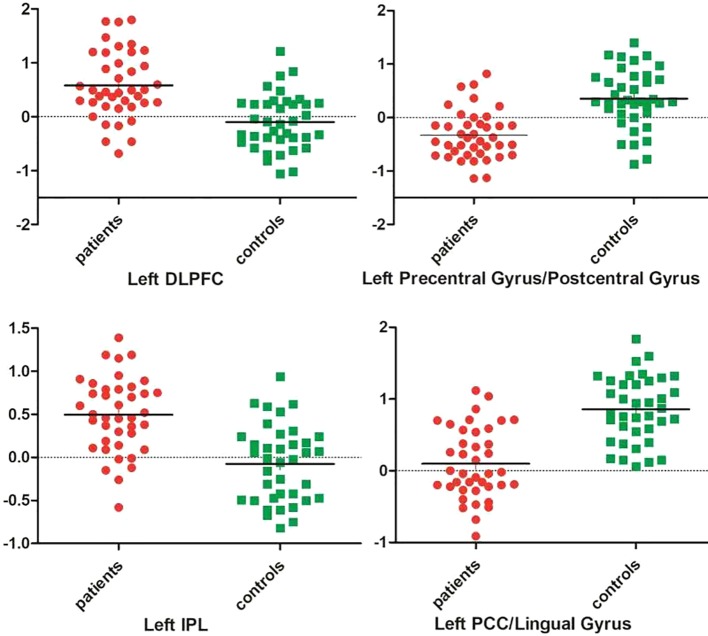
As shown in Table 1 and Figure 1 , patients with OCD showed significantly decreased GFC values in the left PCC/lingual gyrus and left precentral gyrus/postcentral gyrus and significantly increased GFC values in the left DLPFC and left inferior parietal lobule (IPL). A plot of the extracted cluster values with the raw data points of samples (OCD/HCs) was presented in Figure 2 . A sagittal view of the left PCC/lingual gyrus cluster was presented in Supplementary Figure S1 .
Table 1.
Regions with abnormal GFC in the patients with OCD.
| Cluster location | Peak (MNI) | Number of voxels | T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left PCC/Lingual Gyrus | −18 | −66 | 12 | 83 | −5.9634 |
| Left Precentral Gyrus/Postcentral Gyrus | −45 | −9 | 30 | 129 | −5.6116 |
| Left DLPFC | −36 | 45 | 27 | 56 | 5.2824 |
| Left IPL | −54 | −42 | 39 | 83 | 4.3242 |
GFC, global-brain functional connectivity; OCD, obsessive-compulsive disorder; MNI, Montreal Neurological Institute; PCC, posterior cingulate cortex; DLPFC, dorsal lateral prefrontal cortex; IPL, inferior parietal lobule.
Figure 1.
Brain regions with abnormal GFC in patients with OCD. The threshold was set at p < 0.05 corrected by the GRF method. Red and blue denote increased and decreased GFC values respectively. Colour bar indicates t values from two-sample t-tests. L, left side; R, right side; GFC, global-brain functional connectivity; OCD, obsessive-compulsive disorder; GRF, Gaussian Random Field.
Figure 2.
A plot of the extracted cluster values with the raw data points of samples. DLPFC, dorsal lateral prefrontal cortex; IPL, inferior parietal lobule; PCC, posterior cingulate cortex.
There is a debate in whether global signal should be removed. We reanalyzed the data with global signal removal, and obtained the similar results ( Supplementary Table S2 and Supplementary Figure S2 ).
Correlations Between GFC Values and Clinical Variables in Patients With OCD
No correlations were observed between abnormal GFC values and Y-BOCS, HAMD, and HAMA scores in the OCD group with Pearson and Spearman correlation analyses. These correlations were also not significant using the mean FD values and age as covariates with partial correlation analysis.
ROC Analysis in Patients With OCD and HCs
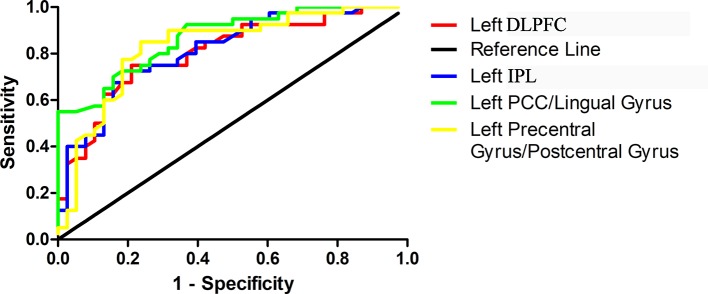
ROC curves were conducted with false positive rate (1-specificity) as X-axis and true-positive rate (sensitivity) as Y-axis and were created by connecting all the points on the graph (each cut-off level generated a point) ( Figure 3 ). Area under the curve (AUC) is an assessment of the entire diagnostic accuracy of the test, and was computed with SPSS 20.0 software (SPSS Inc., Chicago, Illinois, USA). The AUC values of the left PCC/lingual gyrus, left precentral gyrus/postcentral gyrus, left DLPFC, and left IPL were 0.868, 0.830, 0.804, and 0.814, respectively ( Table 2 ).
Figure 3.
Receiver operating characteristic (ROC) curves using the mean GFC values in the left PCC/lingual gyrus, left precentral gyrus/postcentral gyrus, left DLPFC and left IPL to separate patients with OCD from healthy controls. GFC, global-brain functional connectivity; PCC, posterior cingulate cortex; DLPFC, dorsal lateral prefrontal cortex; IPL, inferior parietal lobule.
Table 2.
ROC analysis for differentiating the patients from the controls by using the GFC values.
| Brain regions | Area under the curve | Cut-off point | Sensitivity | Specificity |
|---|---|---|---|---|
| Left PCC/Lingual Gyrus | 0.868 | 0.3776 | 72.50% (29/40) | 84.21% (32/38) |
| Left Precentral Gyrus/Postcentral Gyrus | 0.830 | 0.0664 | 85.00% (34/40) | 76.32% (29/38) |
| Left DLPFC | 0.804 | 0.2552 | 75.00% (30/40) | 78.95% (30/38) |
| Left IPL | 0.814 | 0.2725 | 72.50% (29/40) | 81.58% (31/38) |
ROC, receiver operating characteristic curve; GFC, global-brain functional connectivity; PCC, posterior cingulate cortex; DLPFC, dorsal lateral prefrontal cortex; IPL, inferior parietal lobule.
The results manifested that the GFC value of the left PCC/lingual gyrus could differentiate patients with OCD from HCs with a sensitivity of 72.50% and a specificity of 84.21%. The GFC value of the left precentral gyrus/postcentral gyrus could differentiate patients with OCD from HCs with a sensitivity of 85.00% and a specificity of 76.32%. The GFC value of the left DLPFC could differentiate patients with OCD from HCs with a sensitivity of 75.00% and a specificity of 78.95%. The GFC value of the left IPL could differentiate patients with OCD from HCs with a sensitivity of 72.50% and a specificity of 81.58% ( Table 2 ).
The global balanced accuracies of the left PCC/lingual gyrus, left precentral gyrus/postcentral gyrus, left DLPFC and left IPL were 0.7815 (p < 0.001), 0.7797 (p < 0.001), 0.6826 (p < 0.001), 0.6932 (p < 0.001) respectively for classifying patients with OCD from HCs using the permutation tests.
Discussion
In this study, we investigated the GFC alterations in drug-naive patients with OCD with an unbiased method at rest. The primary results showed that patients with OCD exhibited decreased GFC in the region within the DMN (left PCC/lingual gyrus) and increased GFC in the regions within the ECN (left DLPFC and left IPL), which were consistent with our hypothesis. Moreover, ROC analyses indicated that decreased GFC in the left PCC/lingual gyrus and increased GFC in the left DLPFC and left IPL might be used as candidate neuroimaging markers to differentiate patients with OCD from HCs. Inconsistent with our hypothesis, we found decreased GFC in the region within the sensorimotor network, including the left precentral gyrus/postcentral gyrus, and no significant relationship was observed between abnormal GFC and clinical variables in the OCD group.
Many previous studies have evaluated FC based on assumptive seed definitions with an ROI method, which may acquire different result patterns. Diverse studies typically generate inconsistent results (2). Furthermore, some studies have focused on the networks of interest and ignored the most crucial brain areas involved in the core pathological abnormalities in OCD (12, 13, 15, 28–30). By contrast, in the present research, we used a voxel-wise brain-wide method to focus on FC alterations in drug-naive patients with OCD at rest. Therefore, the present findings were revealed in an unbiased manner and may detect the potential FC abnormalities involved in the core pathological abnormalities of OCD.
The present study found altered GFC in the left PCC/lingual gyrus, left DLPFC, and left IPL, which indicated that there were disrupted functional interactions within the DMN and ECN in patients with OCD. As a neural substrate for human awareness, the PCC/lingual gyrus regulates arousal and attention, and is a crucial brain region in the DMN (31, 32). Reduced FC in the PCC within the DMN was found in previous studies in patients with OCD (28, 29, 33). Reduced negative correlations between the PCC and the fronto-parietal network were discovered in patients with OCD (33). DLPFC and IPL are the core brain regions in the ECN, which are involved in cognitive task switching, response inhibition, and executive planning (34, 35). In our previous study, high regional homogeneity in the DLPFC and IPL (peaking in angular gyrus) and increased FC between DLPFC and IPL at rest in OCD were found with a different sample (12). Decreased GFC in the DMN and increased GFC in the ECN at rest may be related to low self-awareness and focus on controlling external stimuli in patients with OCD. Furthermore, altered GFC in the DMN and ECN may break the balance of the “triple network” model and leads to the difficulty on switching between task-negative and task-positive processing in patients with OCD (8). In conclusion, our findings suggested that disrupted GFC in the DMN and ECN might contribute to the pathophysiology of OCD.
We also observed decreased GFC in regions within the sensorimotor network (i.e., left precentral gyrus/postcentral gyrus), which indicated that altered GFC within the sensorimotor network might be associated with OCD. Patients with OCD have damaged sensory gating and sensory-motor integration, which demonstrate that the deviance of the sensorimotor network may be involved in the inability of patients with OCD to suppress internally repetitive and intrusive thoughts and behavior (36, 37). Meanwhile, the activation in brain regions within the sensorimotor network in the inhibitory control processes may explain the essence of inhibitory control deficits of OCD (38). Furthermore, a recent study revealed that the amplitude of low-frequency fluctuations of the precentral gyrus showed a large discriminative power to identify patients with OCD from HCs (39).
The diagnostic accuracy is high when AUC is above 0.9, medium when AUC is 0.7–0.9, and low when AUC is 0.5–0.7. On this basis, the accuracies of altered GFC values in the left PCC/lingual gyrus, left precentral gyrus/postcentral gyrus, left DLPFC, and left IPL for differentiating patients with OCD from HCs are moderate. Moreover, the sensitivity and specificity of altered GFC values in these brain regions are relatively high. The ROC results are also validated by permutation tests. Therefore, the ROC results of the present study indicate that the decreased GFC in the left PCC/lingual gyrus and left precentral gyrus/postcentral gyrus and increased GFC in the left DLPFC and left IPL may be used as candidate neuroimaging markers for patients with OCD.
Previous studies have reported correlations between abnormal FC and clinical variables, such as obsessive-compulsive severity and illness duration in patients with OCD (2). However, no relationships were found between abnormal GFC and clinical variables in the OCD group in the current study, which indicated that abnormal GFC values might be trait changes independent of clinical variables for patients with OCD (40).
Surprisingly, we did not obtain abnormal GFC values in the regions within the SN. As an important member in the triple network model, the SN filters and detects the external and internal salient information, and has an important role in supervising the interactions between the DMN and ECN (8). In our previous study, decreased FC strength within the SN, between the SN and ECN, and the DMN in OCD at rest was revealed (15). In that study, we only focused on the SN and ignored other important brain regions involved in OCD. However, in the present study, we explored the entire brain FC with an unbiased voxel-wise method, and the present results were related with the core pathological alterations of OCD.
Global brain connectivity (GBC) has been used to study OCD in a few studies. For example, Anticevic et al. found decreased GBC in the left lateral prefrontal cortex and increased GBC in the right putamen and left cerebellar cortex (41). Moreover, weighted degree centrality (DC), a very similar measure to GBC, has been utilized in some studies. Beucke et al. demonstrated higher DC values in the orbitofrontal cortex and basal ganglia (42). Tian et al. discovered increased DC values distributed in the cortico–striato–thalamo-cortical (CSTC) circuits and parietal, occipital, temporal, and cerebellar regions (43). Shin et al. found that changes of DC value in the right ventral frontal cortex were correlated with improvement of obsessive-compulsive symptoms in patients with OCD after SSRIs treatment (16). Gottlich et al. reported that the lower DC value in the bilateral superficial amygdala could predict treatment outcome of cognitive-behavior therapy in OCD (44). The diverse findings may be due to differences across studies such as global signal regression, head motion, and medication use (2, 45).
Another interesting finding in the current study is that all brain regions with altered GFC values are in the left hemisphere. Brain lateralization is implied in the pathogenesis of psychiatric disorders including OCD (46). Previous studies have found that regional alterations of white matter, gray matter volume, and cortical thickness are mainly located in the left hemisphere, including the middle frontal gyrus, IPL, and precentral gyrus, which may be the foundation for GFC abnormalities in the left hemisphere in patients with OCD (47–49). Combining with previous studies and our results, we inferred that GFC abnormalities in the left hemisphere might contribute to the core pathophysiology of OCD. However, several studies have discovered that the changes of structural and functional asymmetry in patients with OCD are mostly distributed to the right hemisphere (50–52). Sample selection including age, sex, illness duration, medication use, comorbidities, and data analysis, including ROI locations, may account for this discrepancy (2).
This study has several limitations apart from the relatively small sample size. First, we did not collect fMRI data with specific tasks, and abnormal GFC at rest might reveal the general pathological changes in OCD. Second, white and gray matter alterations that may underlie the mechanism of GFC are not evaluated in our research. Thirdly, OCD samples were not divided into different subtypes based on different clinical symptoms due to the sample characteristics (i.e., most patients (32/40) are mixed with both obsession and compulsion) and relatively small sample size. Different OCD subtypes may have different GFC patterns at rest (53). Future studies should consider these issues. Finally, the ROIs were first used as differing in OCD vs HCs, and then used in the ROC analysis, which may lead to double dipping. Therefore, the present candidate neuroimaging markers for OCD should be interpreted cautiously.
Despite these limitations, the current study is the first to evaluate voxel-wise brain-wide FC in OCD in an unbiased manner. The results manifest that abnormal FC patterns of the DMN, ECN, and sensorimotor network may account for the pathophysiology of OCD. The current study provides new insights into the alterations in brain organization function in OCD.
Data Availability Statement
All datasets generated for this study are included in the article/ Supplementary Material .
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee at Qiqihar Medical University, China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
PL and WG designed the experiments. GC and YO wrote the manuscript. YC, DL, CJ, ZZ, YW, XM, HC, CL, and ZS performed the experiments. WG, YO, and RY analyzed the data. PL, WG, and XW revised the manuscript. All authors contributed to and have approved the final manuscript.
Funding
This work was supported by grants from Heilongjiang Natural Science Foundation of China (LH2019H064), the Project of Qiqihar Academy of Medical Sciences, China (QMSI2017B-08), the National Key R&D Program of China (2016YFC1307100), the National Natural Science Foundation of China (81771447), Henan Natural Science Foundation Grant (182300410317), and Henan basic and frontier technology research project Foundation Grant (152300410121).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00098/full#supplementary-material
References
- 1. Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol Psychiatry (2010) 15(1):53–63. 10.1038/mp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gürsel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev (2018) 87:151–60. 10.1016/j.neubiorev.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 3. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cognit Sci (2011) 15(10):483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 4. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A (2001) 98(2):676–82. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci (2009) 29(26):8586–94. 10.1523/JNEUROSCI.1868-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang DH, Jang JH, Han JY, Kim JH, Jung WH, Choi JS, et al. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry (2013) 40:340–6. 10.1016/j.pnpbp.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 7. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A (2008) 105(34):12569–74. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, et al. Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J Affect Disord (2017) 223:106–14. 10.1016/j.jad.2017.07.041 [DOI] [PubMed] [Google Scholar]
- 9. Fan J, Zhong M, Zhu X, Gan J, Liu W, Niu C, et al. Resting-state functional connectivity between right anterior insula and right orbital frontal cortex correlate with insight level in obsessive-compulsive disorder. NeuroImage Clin (2017) 15(C):1–7. 10.1016/j.nicl.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koçak OM, Kale E, Çiçek M. Default mode network connectivity differences in obsessive-compulsive disorder. Act Nerv Super (2012) 54(3-4):118–24. 10.1007/bf03379589 [DOI] [Google Scholar]
- 11. Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry (2009) 66(11):1189–200. 10.1001/archgenpsychiatry.2009.152 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Meng X, Hu Q, Cui H, Ding Y, Kang L, et al. Altered resting-state functional organization within the central executive network in obsessive-compulsive disorder. Psychiatry Clin Neurosci (2016) 70(10):448–56. 10.1111/pcn.12419 [DOI] [PubMed] [Google Scholar]
- 13. Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C. Default mode network subsystem alterations in obsessive-compulsive disorder. Br J Psychiatry (2014) 205(5):376–82. 10.1192/bjp.bp.113.137380 [DOI] [PubMed] [Google Scholar]
- 14. Cheng Y, Xu J, Nie B, Luo C, Yang T, Li H, et al. Abnormal resting-state activities and functional connectivities of the anterior and the posterior cortexes in medication-naive patients with obsessive-compulsive disorder. PloS One (2013) 8(6):e67478. 10.1371/journal.pone.0067478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen YH, Li SF, Lv D, Zhu GD, Wang YH, Meng X, et al. Decreased intrinsic functional connectivity of the salience network in drug-naive patients with obsessive-compulsive disorder. Front Neurosci (2018) 12:889. 10.3389/fnins.2018.00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin DJ, Jung WH, He Y, Wang J, Shim G, Byun MS, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry (2014) 75(8):606–14. 10.1016/j.biopsych.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 17. Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishimura T, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry (2011) 26(7):463–9. 10.1016/j.eurpsy.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 18. Li T, Wang Q, Zhang J, Rolls ET, Yang W, Palaniyappan L, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull (2017) 43(2):436–48. 10.1093/schbul/sbw099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui X, Liu F, Chen J, Xie G, Wu R, Zhang Z, et al. Voxel-wise brain-wide functional connectivity abnormalities in first-episode, drug-naive patients with major depressive disorder. Am J Med Genet B Neuropsychiatr Genet (2018) 177(4):447–53. 10.1002/ajmg.b.32633 [DOI] [PubMed] [Google Scholar]
- 20. Ding Y, Ou Y, Su Q, Pan P, Shan X, Chen J, et al. Enhanced global-brain functional connectivity in the left superior frontal gyrus as a possible Endophenotype for schizophrenia. Front Neurosci (2019) 13:145. 10.3389/fnins.2019.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan P, Ou Y, Su Q, Liu F, Chen J, Zhao J, et al. Voxel-based global-brain functional connectivity alterations in first-episode drug-naive patients with somatization disorder. J Affect Disord (2019) 254:82–9. 10.1016/j.jad.2019.04.099 [DOI] [PubMed] [Google Scholar]
- 22. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics (2016) 14(3):339–51. 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- 23. Pruim RHR, Mennes M, Rooij DV, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage (2015) 112:267–77. 10.1016/j.neuroimage.2015.02.064 [DOI] [PubMed] [Google Scholar]
- 24. Parkes L, Fulcher B, Yu CM, Fornitod A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage (2018) 171:415–36. 10.1016/j.neuroimage.2017.12.073 [DOI] [PubMed] [Google Scholar]
- 25. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage (2012) 59(3):2142–54. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu F, Zhu C, Wang Y, Guo W, Li M, Wang W, et al. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clin Neurophysiol (2015) 126(9):1711–6. 10.1016/j.clinph.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 27. Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. (London: Routledge; ) (2013). [Google Scholar]
- 28. Koch K, Reess TJ, Rus OG, Gursel DA, Wagner G, Berberich G, et al. Increased default mode network connectivity in obsessive-compulsive disorder during reward processing. Front Psychiatry (2018) 9:254. 10.3389/fpsyt.2018.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng ZW, Xu T, He QH, Shi CZ, Wei Z, Miao GD, et al. Default network connectivity as a vulnerability marker for obsessive compulsive disorder. Psychol Med (2014) 44(7):1475–84. 10.1017/S0033291713002250 [DOI] [PubMed] [Google Scholar]
- 30. Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, et al. Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp (2017) 38(2):678–87. 10.1002/hbm.23408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex (2007) 17(7):1664–71. 10.1093/cercor/bhl075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci (2011) 31(9):3217–24. 10.1523/JNEUROSCI.5626-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-State functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PloS One (2012) 7(5):e36356. 10.1371/journal.pone.0036356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry (2005) 62(3):301–9. 10.1001/archpsyc.62.3.301 [DOI] [PubMed] [Google Scholar]
- 35. Kwon JS, Jang JH, Choi JS, Kang DH. Neuroimaging in obsessive-compulsive disorder. Expert Rev Neurother (2009) 9(2):255–69. 10.1586/14737175.9.2.255 [DOI] [PubMed] [Google Scholar]
- 36. Russo M, Naro A, Mastroeni C, Morgante F, Terranova C, Muscatello MR, et al. Obsessive-compulsive disorder: a “sensory-motor” problem? Int J Psychophysiol (2014) 92(2):74–8. 10.1016/j.ijpsycho.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 37. Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology (2012) 37(5):1216–23. 10.1038/npp.2011.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morein-Zamir S, Voon V, Dodds CM, Sule A, van Niekerk J, Sahakian BJ, et al. Divergent subcortical activity for distinct executive functions: stopping and shifting in obsessive compulsive disorder. Psychol Med (2016) 46(4):829–40. 10.1017/S0033291715002330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bu X, Hu X, Zhang L, Li B, Zhou M, Lu L, et al. Investigating the predictive value of different resting-state functional MRI parameters in obsessive-compulsive disorder. Transl Psychiatry (2019) 9(1):17. 10.1038/s41398-018-0362-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo W, Yao D, Jiang J, Su Q, Zhang Z, Zhang J, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry (2014) 49(2):16–20. 10.1016/j.pnpbp.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 41. Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry (2014) 75(8):595–605. 10.1016/j.biopsych.2013.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry (2013) 70(6):619–29. 10.1001/jamapsychiatry.2013.173 [DOI] [PubMed] [Google Scholar]
- 43. Tian L, Meng C, Jiang Y, Tang Q, Wang S, Xie X, et al. Abnormal functional connectivity of brain network hubs associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder: a resting-state functional MRI study. Prog Neuropsychopharmacol Biol Psychiatry (2016) 66:104–11. 10.1016/j.pnpbp.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 44. Gottlich M, Kramer UM, Kordon A, Hohagen F, Zurowski B. Resting-state connectivity of the amygdala predicts response to cognitive behavioral therapy in obsessive compulsive disorder. Biol Psychol (2015) 111:100–9. 10.1016/j.biopsycho.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 45. Li J, Kong R, Liegeois R, Orban C, Tan Y, Sun N, et al. Global signal regression strengthens association between resting-state functional connectivity and behavior. NeuroImage (2019) 196:126–41. 10.1016/j.neuroimage.2019.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peng Z, Li G, Shi F, Shi C, Yang Q, Chan RC, et al. Cortical asymmetries in unaffected siblings of patients with obsessive-compulsive disorder. Psychiatry Res (2015) 234(3):346–51. 10.1016/j.pscychresns.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shin YW, Yoo SY, Lee JK, Ha TH, Lee KJ, Lee JM, et al. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp (2007) 28(11):1128–35. 10.1002/hbm.20338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, et al. Grey matter abnormalities in obsessive-compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Br J Psychiatry (2001) 179:330–4. 10.1192/bjp.179.4.330 [DOI] [PubMed] [Google Scholar]
- 49. Fan Q, Yan X, Wang J, Chen Y, Wang X, Li C, et al. Abnormalities of white matter microstructure in unmedicated obsessive-compulsive disorder and changes after medication. PloS One (2012) 7(4):e35889. 10.1371/journal.pone.0035889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Menzies L, Williams GC, Chamberlain SR, Ooi C, Fineberg N, Suckling J, et al. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am J Psychiatry (2008) 165(10):1308–15. 10.1176/appi.ajp.2008.07101677 [DOI] [PubMed] [Google Scholar]
- 51. Rao NP, Arasappa R, Reddy NN, Venkatasubramanian G, Reddy YCJ. Lateralisation abnormalities in obsessive-compulsive disorder: a line bisection study. Acta Neuropsychiatr (2015) 27(4):242–7. 10.1017/neu.2015.23 [DOI] [PubMed] [Google Scholar]
- 52. Narayan VM, Narr KL, Phillips OR, Thompson PM, Toga AW, Szeszko PR. Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport (2008) 19(15):1551–5. 10.1097/WNR.0b013e3283112720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ravindran A, Richter M, Jain T, Ravindran L, Rector N, Farb N. Functional connectivity in obsessive-compulsive disorder and its subtypes. Psychol Med (2019), 1–9. 10.1017/s0033291719001090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/ Supplementary Material .