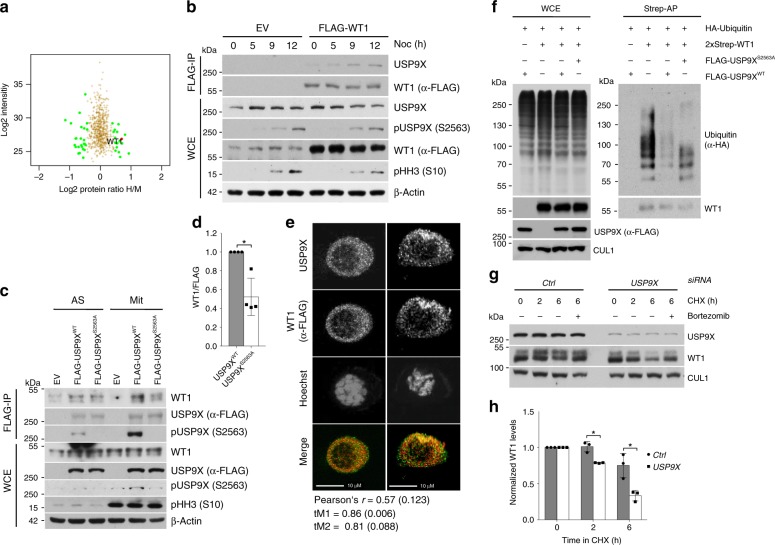
Fig. 2. WT1 is a substrate of pUSP9X (serine 2563) in mitosis.
a Mass spectrometric analysis of the USP9X-dependent ubiquitome in mitotic HEK 293T cells. USP9X knockdown cells were cultured in heavy (“H”), control knockdown cells in medium (“M”) SILAC media. b Co-immunoprecipitation of FLAG-tagged WT1 with endogenous USP9X from HEK 293T cells (EV, expression vector). Cells were exposed to nocodazole, collected, lysed (WCE, whole cell extracts), and subjected to anti-FLAG immunoprecipitation before analysis by western blot. c Co-immunoprecipitation of USP9XWT rather than USP9XS2563A with WT1. Expression vector (EV), FLAG-tagged USP9XWT or USP9XS2563A was overexpressed in asynchronous (AS) or mitotically arrested (Mit, 15 h nocodazole) HEK 293T cells, and purified by immunoprecipitation before western blot analysis. d Quantification of WT1 co-immunoprecipitated with either USP9XWT or USP9XS2563A from n = 4 biologically independent experiments as described in c. Ratio paired t-test was applied with *p = 0.0248. e Immunofluorescence imaging of mitotic U2OS cells showing colocalization of WT1 (red) and USP9X (green) in two representative mitotic cells. Cells were transfected with FLAG-tagged WT1 and arrested in mitosis using nocodazole (15 h) before fixing and staining. Values for Pearson’s and Manders’ coefficients (tM1 and tM2) are the mean and SD calculated from n = 17 biologically independent cells. Scale bar, 10 µm. f In vivo ubiquitylation assay showing pUSP9X-dependent ubiquitylation of WT1. HEK 293T cells transfected with FLAG-tagged USP9XWT or USP9XS2563A, 2xStrep-tagged WT1, and HA-tagged Ubiquitin were synchronized in mitosis using nocodazole. Fourteen hours before collection nocodazole, bortezomib, and the caspase inhibitor Z-VAD-FMK were added. Cells were lysed under denaturing conditions and 2xStrep affinity purification was performed. g Cycloheximide time course showing USP9X-dependent WT1 stability. U2OS cells were treated with control or USP9X siRNA and arrested in mitosis using sequential thymidine and nocodazole (11 h). Mitotic cells were collected by shake-off and cycloheximide, bortezomib (where indicated), and Z-VAD-FMK added before collection and western blot analysis. h Quantification of WT1 protein levels from n = 3 biologically independent experiments as described in g. Western blot bands of WT1 were quantified using ImageJ software and normalized to loading control and time point 0. One sample t-test was applied with *p = 0.0219; *p = 0.0389. Throughout this figure mean and standard deviations as error bars are displayed.