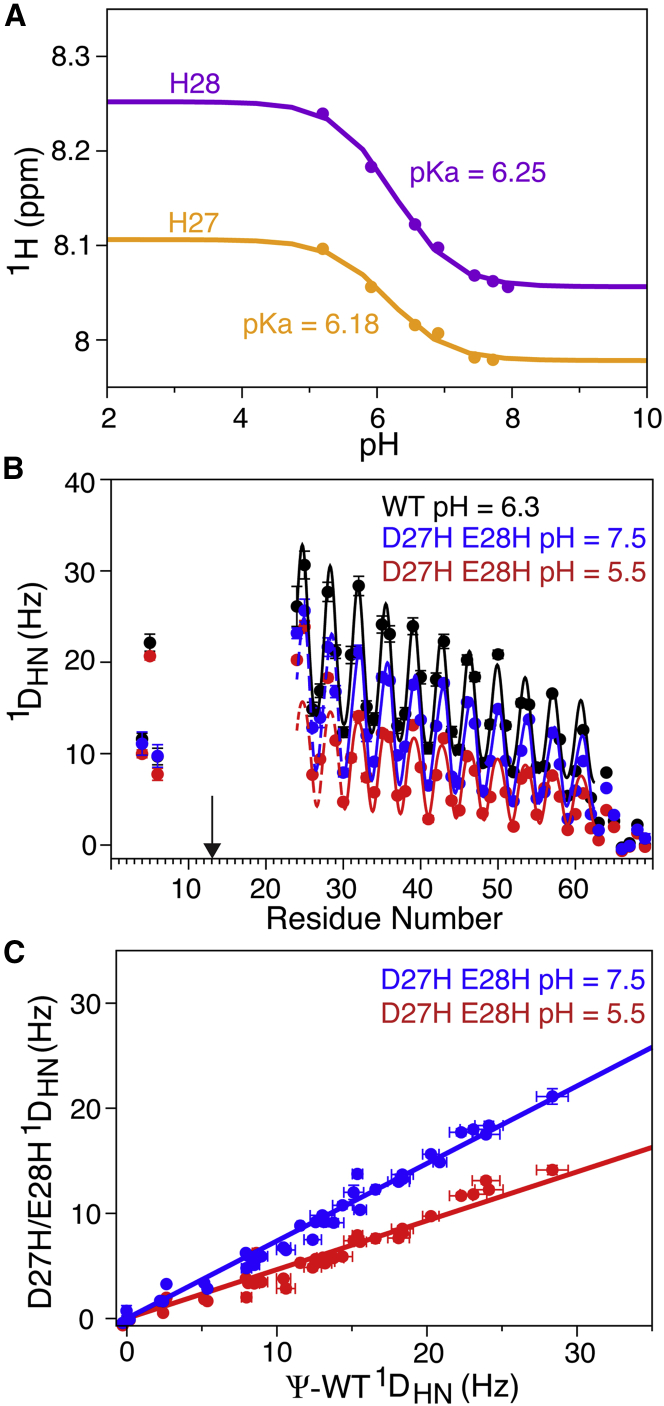
Figure 7.
pH dependence of the helical stiffness of the D27H/E28H double mutant of the Ψ-WT SAH domain of myosin VI, paramagnetically tagged at I13C. (A) Shown here is the 1HN chemical shift titration of H27 (yellow) and H28 (magenta), with the solid lines representing the best fits to the Henderson-Hasselbalch equation. (B) RDC versus residue number in the Ψ-WT (black) and D27H/E28H mutants at pH 7.5 (blue) and pH 5.5 (red) is shown here. Solid lines correspond to the best-fitted dipolar wave patterns (28) for residues R24–E62, multiplied by a decaying exponential function with a decay constant of 49.75 residues. For the D27H/E28H mutants, only RDCs for residues R30–E62 were included in the dipolar wave fit; continuation of the dipolar wave in the N-terminal direction is depicted by dashed lines. The arrow marks the location of the paramagnetic tag. (C) The correlation between 1DNH RDCs of residues R30–E68 in the D27H/E28H double mutant and Ψ-WT at pH 7.5 (blue, slope 0.74; R2 = 0.985) and pH 5.5 (slope 0.47; R2 = 0.976) is shown here. To see this figure in color, go online.