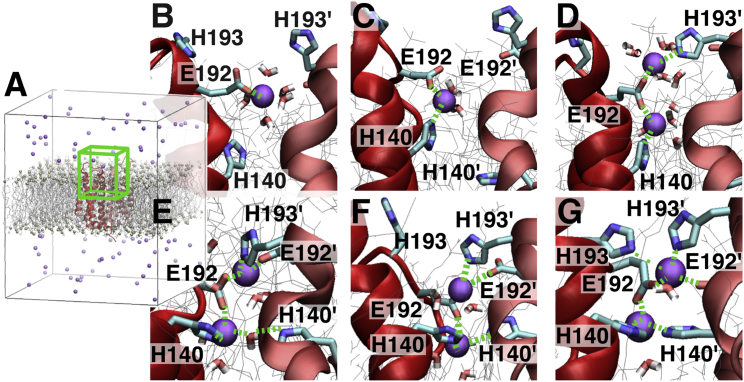
Figure 5.
Time evolution of the binding in DimerM (MD 7 of DimerM⋅180⋅WT⋅freeZn in Table S1). (A) The periodic box for the whole system is shown. (B–G) Snapshots during the time course of the simulation are shown. The region of the system corresponding to the green box in (A) is shown. The zinc cations are depicted as violet balls. Water molecules have been removed for clarity except for those within 3 Å of Zn2+, which are shown as dynamic bonds. The snapshots are taken at (B) 0.6 ns, (C) 0.8 ns, (D) 1.7 ns, (E) 5.9 ns, (F) 75 ns, and (G) 1420 ns of the trajectory. A first Zn2+ ion is coordinated by the glutamate 192 residue of one HV1 subunit (B), then by the histidine 140 residue (Zn2) of the same subunit (C). Then, a second Zn2+ is coordinated by E192 and H193′ residues (Zn1) of the other HV1 subunit (D). A reorganization of the binding sites then takes place: the first Zn2+ ion (Zn2) is further coordinated by the second histidine, H140′, first via a water-mediated interaction (E) and then directly (F), while the second (Zn1) is further coordinated by E192′ (E). The coordination spheres in (F) remain stable for the rest of the simulation, up to 3000 ns. The participation of H193 in the binding is transient but recurrent in the rest of the simulation (G). The binding sites in (G) are closely similar to the results of DimerM⋅2Zn (Fig. 4; Table S2).