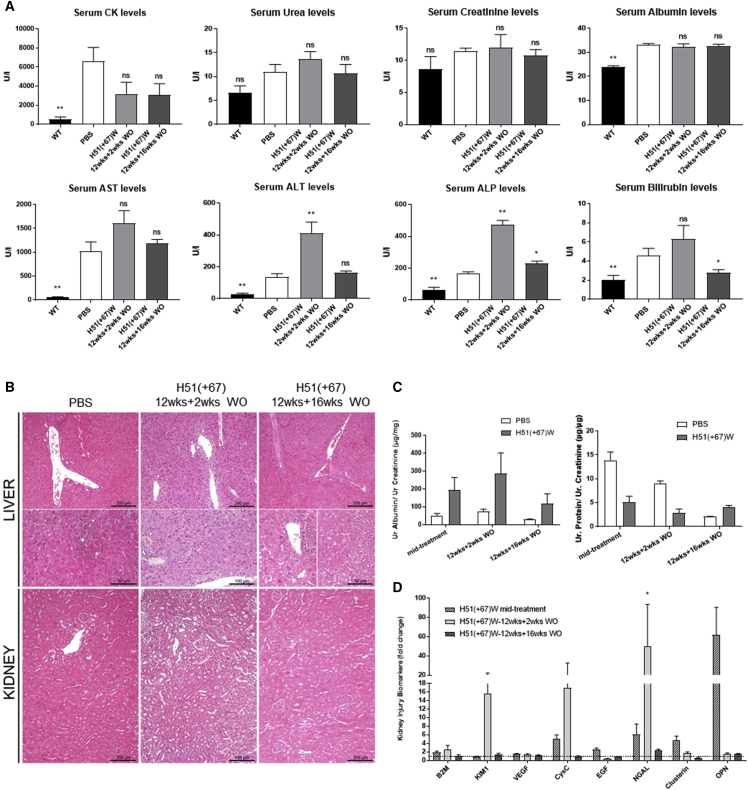
Figure 7.
High Dose of H51(+67)W for 12 Weeks Induces Mild and Reversible Renal and Hepatic Toxicity
(A) Serum biochemistry was analyzed 2 weeks (n = 4) or 16 weeks (n = 4) after the end of the 12-week treatment at 200 mg/kg/week (wild type [WT] n = 6, PBS n = 6). (B) H&E staining of liver and kidney sections from H51(+67)W-treated mdx52 mice, analyzed 2 weeks or 16 weeks after the end of the 12-week treatment at 200 mg/kg compared to PBS-treated mdx52 mice (PBS). Histopathological analysis revealed: (left) rare inflammatory foci in the liver of control mice with minimal anisocytosis, anisokaryosis, vacuolation, and steatosis; (middle) diffuse chronic hepatitis with multifocal necrosis of hepatocytes and almost no lesion in the kidney, 2 weeks after the end of H51(+67)W treatment; (right) rare inflammatory foci in the liver, associated with multifocal anisocytosis, anisokaryosis, and vacuolation of hepatocytes with steatosis and almost no lesion in the kidney after the 16-week recovery period. (C) Urinary albumin/creatinine (left) and protein/creatinine (right) ratios from mouse urine samples collected during treatment (H51(+67)W midtreatment, n = 4) or 2 weeks (H51(+67)W 12wks+2wks WO, n = 4) or 16 weeks (H51(+67)W 12wks+16wks WO, n = 4) after the end of the 12-week treatment. (D) KIBs were evaluated in urine samples from controls and treated mdx mice collected during treatment (H51(+67)W midtreatment, n = 4) and 2 weeks (H51(+67)W-12wks+2wks WO, n = 4) or 16 weeks (H51(+67)W-12wks+16wks WO, n = 4) after the end of the 12-week treatment tcDNA using Luminex technology. KIB levels were normalized to unit of creatinine (UCR) and expressed as fold-change ratio of their age-matched PBS controls (n = 3). Results are expressed as mean ± SEM; not significant (ns) = p > 0.05, *p < 0.05, **p < 0.01 compared to PBS.