Abstract
Background
Plasma volume status (PVS) has been evaluated recently as a prognostic marker of acute heart failure (AHF). However, whether evaluating PVS alone is sufficient remains unclear.
Methods
Of 675 patients with AHF screened, 601 were enrolled. The PVS, prognostic nutritional index (PNI) (lower = worse), and Controlling Nutritional Status (CONUT) score (higher = worse) were evaluated. Patients were divided into 2 groups according to PVS value (low- or high-PVS group) and were further subdivided into 4 groups (low- or high-PVS/CONUT group and low- or high-PVS/PNI group).
Results
A Kaplan–Meier curve showed a significantly lower survival rate in the high-PVS group than in the low-PVS group, the high-PVS/high-CONUT group than in the high-PVS/low-CONUT group, and the high-PVS/low-PNI group than in the high-PVS/high-PNI group. A multivariate Cox regression model showed that high PVS (hazard ratio [HR], 1.642; 95% confidence interval [CI], 1.049-2.570) and high PVS/high CONUT (HR, 2.076; 95% CI, 1.147-3.757) and high PVS/low PNI (HR, 2.094; 95% CI, 1.166-3.761) were independent predictors of 365-day mortality.
Conclusions
An adverse outcome was predicted by the evaluation of PVS; furthermore, a malnutrition status with a high PVS leads to an adverse outcome. The simultaneous evaluation of nutrition status and PVS is essential to predict an AHF outcome.
Highlights
-
•
Whether evaluating plasma volume status (PVS) alone is sufficient remains unclear in acute heart failure.
-
•
A multivariate Cox regression model showed that high PVS and high PVS/high Controlling Nutritional Status and high PVS/low prognostic nutritional index were independent predictors of 365-day mortality.
-
•
A malnutrition status with a high PVS leads to an adverse outcome.
-
•
The simultaneous evaluation of nutrition status and PVS is essential to predict an acute heart failure outcome.
Résumé
Contexte
La valeur pronostique du volume plasmatique (VP) dans l’insuffisance cardiaque aiguë (ICA) a récemment été évaluée. On ne sait toutefois pas si le volume plasmatique à lui seul peut suffire.
Méthodologie
Sur les 675 patients présentant une ICA diagnostiquée, 601 ont été retenus. Le VP, l’indice nutritionnel pronostique (PNI; plus l’indice est faible, plus l’état nutritionnel est mauvais) et le score CONUT (Controlling Nutritional Status, contrôle de l’état nutritionnel; plus le score est élevé, plus l’état nutritionnel est mauvais) ont été évalués. Les patients ont été répartis en deux groupes en fonction du VP (VP faible et VP élevé), puis de nouveau en quatre sous-groupes (VP/CONUT faible ou élevé et VP/PNI faible ou élevé).
Résultats
La courbe de Kaplan-Meier montre que le taux de survie est significativement inférieur dans le groupe VP élevé par rapport au groupe VP faible, dans le groupe VP élevé/score CONUT élevé par rapport au groupe VP élevé/score CONUT faible, et dans le groupe VP élevé/PNI faible par rapport au groupe VP élevé/PNI élevé. L’analyse au moyen d’un modèle de régression de Cox multivarié a révélé qu’un VP élevé (rapport des risques instantanés [RRI] de 1,642; intervalle de confiance [IC] à 95 % : de 1,049 à 2,570), un VP élevé assorti d’un score CONUT élevé (RRI de 2,076; IC à 95 % : de 1,147 à 3,757) et un VP élevé assorti d’un PNI faible (RRI de 2,094; IC à 95 % : de 1,166 à 3,761) étaient des facteurs prédictifs indépendants de la mortalité à 365 jours.
Conclusions
L’évaluation du VP a permis de prédire une issue défavorable; en outre, les données montrent qu’un état de malnutrition conjugué à un VP élevé est un facteur de mauvais pronostic. Il est essentiel d’évaluer simultanément l’état nutritionnel et le VP pour prédire l’ICA.
The features of acute heart failure (AHF) may differ. Sudden-onset pulmonary edema with high systolic blood pressure is categorized as “vascular” failure or “hypertensive” heart failure (HF), whereas HF with the gradual development of symptoms over days is characterized as “cardiac” failure or “normotensive–hypotensive” HF.1, 2, 3 Therefore, volume status varies among individuals.4 Volume expansion, that is, systemic venous congestion, sometimes leads to adverse outcomes in patients with HF.5 In the 2010s, the noninvasive evaluation of plasma volume (PV) was explored in patients with chronic HF or AHF, and the clinical implications of the calculated or estimated plasma volume status (PVS) were described.6, 7, 8, 9
The importance of evaluating nutritional status also has been suggested in patients with HF.10 Because malnutrition is a major problem in an aging society,11 HF guidelines recommend the evaluation of nutritional status.2,3 Previous research evaluating nutrition status in patients with AHF using various tools (ie, albumin, total cholesterol, Controlling Nutritional Status [CONUT] score, and prognostic nutritional index [PNI]) has indicated that malnutrition is an independent predictor of mortality, HF progression, HF events, and adverse outcomes.12, 13, 14, 15, 16 We also previously reported the importance of malnutrition in patients with severely decompensated AHF.17 However, the association between the nutritional status and the degree of general congestion/PV has not been adequately explored.
Volume overload/expansion due to malnutrition was hypothesized to have a prognostic impact in patients with AHF. However, patients with volume overload may not have a poor prognosis if their nutrition status is good. Therefore, the prognostic efficacy of PVS alone and PVS with the PNI and the CONUT score was examined in patients with AHF.
Methods
Patients
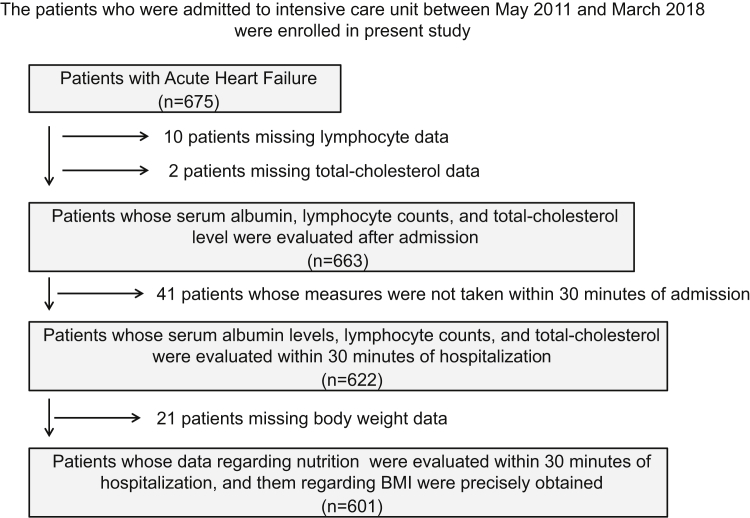
A total of 675 patients with AHF admitted to the intensive care unit (ICU) of Nippon Medical School Chiba Hokusoh Hospital (Chiba, Japan) between May 2011 and March 2018 were screened. Of these, 12 patients who lacked serum albumin, lymphocyte count, or total cholesterol data were excluded from this study. An additional 41 patients for whom these examinations were not performed within 30 minutes of hospitalization were excluded. Finally, 21 patients missing body weight data in their hospital medical records were also excluded. Ultimately, 601 patients with AHF were enrolled in this study (Fig. 1).
Figure 1.
Patient selection process. Between May 2011 and March 2018, 1412 patients who were admitted to the intensive care unit (ICU) at Nippon Medical School Chiba Hokusoh Hospital were screened. Of these, 12 patients who lacked serum albumin, lymphocyte counts, or total cholesterol measures were excluded. A further 41 for whom these measures were not obtained within 30 minutes of admission and 21 for whom the data of body weight were missed were also excluded, leaving 601 patients with acute heart failure (AHF) who were enrolled in the study. BMI, body mass index.
AHF is defined as a gradual or rapid change in HF signs and symptoms requiring urgent therapy. HF was diagnosed on the basis of clinical history (ie, symptoms, functional limitation, prior cardiac disease, risk factors, exacerbating factors, comorbidities, and drugs), physical examination (ie, of vital signs, weight and volume status of the heart, lungs, abdomen, and peripheral vascular regions), and initial investigations (ie, chest radiography, 12-lead electrocardiography, laboratory measurements of troponins, blood urea nitrogen,18 creatinine, sodium, potassium, glucose, liver function, and complete blood count). Furthermore, evaluations of plasma natriuretic peptide and echocardiography were performed to support the diagnosis of HF. The treating physician in the emergency department diagnosed AHF according to the aforementioned procedure within 30 minutes of admission.2,3 All patients had a New York Heart Association (NYHA) functional class of III or IV.
Furthermore, all included patients received treatment with diuretics or vasodilators for AHF. The patients who needed 1 of the following 3 treatments required intensive care: (1) high-flow oxygen therapy (including mechanical support) to treat orthopnea; (2) inotropes or mechanical support due to low blood pressure; or (3) various types of diuretics to improve general or lung edema. Patients with HF caused by ST-T segment elevation acute coronary syndrome were excluded from the study. The physician selected the treatment strategy.
Blood sample measurements and data collection
Blood samples from the included patients were collected within 30 minutes of admission. The samples were centrifuged within 5 minutes of collection at 4°C, immediately frozen, and stored at –80°C until analysis. The data were retrospectively retrieved from the hospital medical records.
Patient characteristics, including age, sex, presence of de novo or recurrent HF, etiology of HF, risk factors for atherosclerosis (diabetes mellitus, hypertension, and dyslipidemia), vital signs (systolic blood pressure and heart rate), left ventricular ejection fraction (LVEF) on echocardiograms, arterial blood gas data, laboratory data (eg, blood urea nitrogen,18 total bilirubin, hemoglobin, brain natriuretic peptide, C-reactive protein [CRP]), medications administered during admission to the ICU, and duration of admission (duration of ICU stay and hospital stay) were compared.
The LVEF was calculated using the Teichholz method or Simpson’s method at admission (Sonos 5500; Hewlett Packard, Palo Alto, CA; or Vivid I; GE Yokogawa Medical, Tokyo, Japan). Because the LVEF was measured during the acute phase, it was not adequately evaluated because of severe orthopnea. The methodology for the LVEF measurement (Teichholz method or Simpson’s method) was decided on a case-by-case basis.
Procedures and prognosis
PVS was calculated using the following formula: ([actual PV − ideal PV]/ideal PV) × 100(%). The actual and ideal PV were defined as follows: actual PV = (1 – hematocrit) × (a + [b × body weight]) (a = 1530 in male and 864 in female patients; b = 41.0 in male and 47.9 in female patients), ideal PV = c × body weight (c = 39 in male and 40 in female patients).9
The PNI was calculated using the following formula: 10 × serum albumin (g/dL) + 0.005 × lymphocyte (/μL) (lower = worse).19 The CONUT score was calculated using a scoring system consisting of serum albumin, lymphocytes, and total cholesterol (range, 0-12; higher = worse).20 Patients were divided into 4 groups (severe, moderate, mild, and normal) based on their serum albumin, lymphocyte, and total cholesterol levels: serum albumin ≥ 3.5 g/dL (normal), 3 to 3.49 (mild), 2.5 to 2.99 (moderate), and < 2.5 (severe); lymphocytes ≥ 1600/μL (normal), 1200 to 1599 (mild), 800 to 1199 (moderate), and < 800 (severe); and total cholesterol ≥ 180 mg/dL (normal), 140 to 179 (mild), 100 to 139 (moderate), and < 100 (severe). The median PNI and CONUT scores established the cutoff values for the low and high groups: 42.33 for the PNI (low PNI: PNI < 42.30; high PNI: PNI ≥ 42.30) and 3 for the CONUT score (low: CONUT score ≤ 3; high: CONUT score ≥ 4.0).
Patients were divided into 2 groups according to the PVS value (low PVS: PVS ≤ 12.0%, n = 300; high PVS: PVS > 12.0%, n = 301). The median PVS established the cutoff value for the low and high groups. Subsequently, patients were further subdivided into 4 groups according to nutritional status and PVS. By using the CONUT score, patients were divided as follows: low-PVS/low-CONUT group (n = 189), low-PVS/high-CONUT group (n = 111), high-PVS/low CONUT group (n = 141), and high-PVS/high-CONUT group (n = 160). By using the PNI, patients were divided as follows: low-PVS/high-PNI group (n = 180), low-PVS/low-PNI group (n = 120), high-PVS/high-PNI group (n = 121), and high-PVS/low-PVS group (n = 180).
Long-term prognosis, including all-cause death within 365 days, was evaluated. Patients had clinical follow-ups at routine outpatient visits. The prognoses of patients being followed up at other institutes were determined through telephone interviews. The prognosis of 365-day mortality and HF events was evaluated using the Cox proportional hazards regression model and Kaplan–Meier curve analysis.
Statistical analyses
All data were analyzed using the SPSS 22.0 software program (SPSS Japan Institute, Tokyo, Japan). All numerical data were expressed as the median and range or interquartile range, according to normality. We used the Shapiro–Wilk W-test to assess normality. The Mann–Whitney U test was used for comparisons between the 2 groups. The chi-square test was used to compare proportions. P values < 0.05 indicated statistical significance.
The prognostic value of PVS (high vs low PVS) and nutrition status and PVS (by low or high PVS/PNI or low or high PVS/CONUT) vs a reference group of normal patients (low PVS/low CONUT and low PVS/high PNI) was assessed using a multivariate Cox proportional hazards regression model. A multivariate Cox regression analysis was performed to determine the hazard ratio (HR) for 365-day mortality. The cumulative survival rates in the 4 groups were analyzed using Kaplan–Meier curves, and a log-rank test was used to calculate the statistical significance of the differences.
Ethical review
The Research Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital approved the study protocol. Because of the retrospective design of the study, written informed consent was waived, in accordance with the guidance provided by the Ethics Committee.
Results
Patient characteristics and differences between PVS groups
The study population had a median age of 76 years; 65.4% were male with a median age of 76 years. During the initial evaluation, it was determined that 219 patients (36.4%) had been previously hospitalized for HF. Within the total cohort, 240 patients (39.9%) had ischemic heart disease, and 361 patients (60.1%) had nonischemic heart disease, including cardiomyopathy (hypertrophic cardiomyopathy [n = 16], dilated cardiomyopathy [n = 50], drug-induced cardiomyopathy [n = 1] and sarcoidosis [n = 1]), hypertensive heart disease (n = 92), valvular disease (n = 144), and other heart diseases (n = 57). Most patients were NYHA class IV (n = 468, 77.9%), and the median LVEF on admission was 39.0%.
The high-PVS group included fewer men and more readmitted patients. Patients in this group were significantly older, had significantly more LVEF preservation, and had heart rates that were significantly lower than those reported in the low-PVS group. Furthermore, the levels of serum total bilirubin and hemoglobin were significantly lower in the high-PVS group vs the low-PVS group. In contrast, the levels of blood urea nitrogen, creatinine, CRP, and brain natriuretic peptide were significantly higher in the high-PVS group vs the low-PVS group (Table 1).
Table 1.
Characteristics of patients by the difference in PVS
| Total (n = 601) | PVS |
P value | ||
|---|---|---|---|---|
| Low |
High |
|||
| (n = 300) | (n = 301) | |||
| Status and vital signs | ||||
| Age (y) | 76 (67-82) | 71 (62-78) | 80 (72-85) | < 0.001 |
| Gender (male, %) | 393 (65.4%) | 242 (80.7%) | 157 (50.2%) | < 0.001 |
| Type (readmission, %) | 219 (36.4%) | 92 (30.7%) | 127 (42.2%) | 0.004 |
| LVEF (%) | 39 (27-51) | 34 (25-50) | 42 (30-55) | < 0.001 |
| LVEF ≤ 40% (yes, %) | 332 (55.2%) | 190 (63.3%) | 142 (47.2%) | < 0.001 |
| NYHA (IV, %) | 468 (77.9%) | 239 (79.7%) | 229 (76.1%) | 0.326 |
| Systolic blood pressure (mm Hg) | 158 (122-183) | 162 (122-189) | 152 (123-180) | 0.048 |
| Pulse (beats/min) | 107 (88-125) | 110 (94-130) | 102 (84-119) | < 0.001 |
| Etiology | ||||
| Ischemia (yes, %) | 240 (39.9%) | 124 (41.3%) | 116 (38.5%) | 0.506 |
| Medical history | ||||
| Hypertension (yes, %) | 452 (75.2%) | 216 (72.0%) | 236 (78.4%) | 0.073 |
| Diabetes mellitus (yes, %) | 292 (48.6%) | 139 (46.3%) | 153 (50.8%) | 0.289 |
| Dyslipidemia (yes, %) | 308 (51.2%) | 156 (52.0%) | 152 (50.5%) | 0.744 |
| Arterial blood gas | ||||
| pH | 7.36 7.24-7.43) | 7.36 (7.22-7.43) | 7.36 (7.26-7.43) | 0.176 |
| PCO2 (mm Hg) | 38.8 32.7-52.6) | 40.1 (33.7-54.3) | 37.8 (32.3-49.8) | 0.060 |
| PO2 (mm Hg) | 98.7 (71.2-145.0) | 92.4 (71.1-136.0) | 105.8 (71.5-155.0) | 0.076 |
| HCO3- (mmol/L) | 21.8 19.2-24.3) | 22.1 (19.5-24.5) | 21.3 (18.4-24.1) | 0.065 |
| SaO2 (%) | 97 (93-99) | 96 (93-98) | 97 (93-99) | 0.046 |
| Lactate (mmol/L) | 1.8 (1.2-3.5) | 1.8 (1.2-3.7) | 1.8 (1.1-3.2) | 0.081 |
| Laboratory data | ||||
| Total bilirubin (μmol/L) | 10.3 (6.8-17.1) | 12.0 (8.6-18.8) | 8.6 (6.8-13.7) | < 0.001 |
| Uric acid (μmol/L) | 410 (321-482) | 416 (333-494) | 399 (309-476) | 0.029 |
| Sodium (mmol/L) | 140 (137-142) | 140 (137-142) | 140 (137-142) | 0.705 |
| Potassium (mmol/L) | 4.3 (3.9-4.8) | 4.3 (4.0-4.8) | 4.3 (3.8-4.7) | 0.296 |
| Hemoglobin (g/L) | 122 (103-137) | 137 (126-149) | 105 (92-118) | < 0.001 |
| BUN (μmol/L) | 9.4 (6.5-15.2) | 8.9 (6.0-12.1) | 11.6 (7.3-17.6) | < 0.001 |
| Creatinine (μmol/L) | 107 (78-185) | 100 (76-147) | 120 (79-229) | < 0.001 |
| CRP (μg/L) | 8200 (2300-3680) | 7250 (2475-30,200) | 8700 (2100-47,500) | 0.437 |
| BNP (pmol/L) | 34.5 (15.7-159.4) | 26.9 (12.6-468.4) | 41.2 (20.1-134.8) | < 0.001 |
| Nutritional status | ||||
| PNI | 42.3 (37.2-48.8) | 44.8 (39.1-50.9) | 40.2 (35.2-46.2) | < 0.001 |
| CONUT score | 3 (1-5) | 3 (1-4) | 4 (2-6) | < 0.001 |
| Albumin (g/L) | 35 (32-38) | 36 (33-39) | 34 (31-37) | < 0.001 |
| Lymphocyte count (/μL) | 1400 (721-2358) | 1570 (879-2694) | 1159 (621-1952) | < 0.001 |
| Total cholesterol (mmol/L) | 4.24 (3.57-5.04) | 4.34 (3.65-5.26) | 4.16 (3.47-4.89) | 0.007 |
| Medication (cases) during ICU | ||||
| Furosemide (yes, %) | 541 (90.0%) | 269 (89.7%) | 272 (90.4%) | 0.787 |
| Nitroglycerin (yes, %) | 250 (41.6%) | 122 (40.7%) | 128 (42.5%) | 0.679 |
| Nicorandil (yes, %) | 95 (15.8%) | 43 (14.3%) | 52 (17.3%) | 0.371 |
| Carperitide (yes, %) | 221 (36.8%) | 112 (37.3%) | 109 (36.2%) | 0.800 |
| Dopamine (yes, %) | 38 (6.3%) | 19 (6.3%) | 19 (6.3%) | 1.000 |
| Dobutamine (yes, %) | 132 (22.0%) | 68 (22.7%) | 64 (21.3%) | 0.694 |
| ACE-I/ARB (yes, %) | 182 (30.3%) | 108 (36.0%) | 74 (24.6%) | 0.030 |
| β-Blocker (yes, %) | 171 (28.5%) | 101 (33.7%) | 70 (23.3%) | 0.003 |
| Spironolactone (yes, %) | 215 (35.8%) | 131 (43.7%) | 84 (27.9%) | < 0.001 |
| Statin (yes, %) | 182 (30.3%) | 96 (32.0%) | 86 (28.6%) | 0.376 |
| Outcome | ||||
| ICU hospitalization (d) | 4 (3-6) | 4 (3-6) | 4 (3-6) | 0.217 |
| Total hospitalization (d) | 24 (16-41) | 23 (15-38) | 27 (16-45) | 0.122 |
| In-hospital mortality (yes, %) | 71 (11.8%) | 28 (9.3%) | 43 (14.3%) | 0.076 |
P values between the low-PVS and high-PVS groups were determined using the Mann–Whitney U test or chi-square test. All numerical data are expressed as the median (25%-75% interquartile range).
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CONUT, Controlling Nutritional Status; CRP, C-reactive protein; ICU, intensive care unit; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PNI, prognostic nutritional index; PVS, plasma volume status.
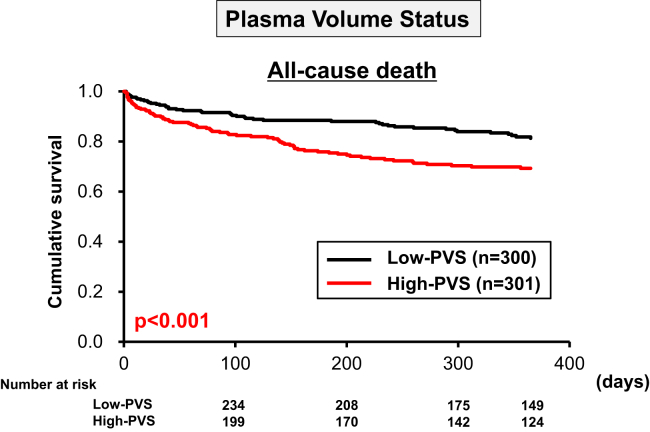
The Kaplan–Meier survival curves, including all-cause death within 365 days, for the 4 PVS groups are shown in Figure 2. The survival rates in the high-PVS group were significantly lower than those in the low-PVS group. The multivariate Cox proportional hazards regression model showed that high PVS was an independent predictor of 365-day mortality in patients with AHF (HR, 1.642; 95% confidence interval [CI], 1.049-2.570; P = 0.030) (Table 2).
Figure 2.
Kaplan–Meier survival curves for PVS. Kaplan–Meier survival curves showed that the prognosis, including all-cause death, was significantly poorer in the high-PVS group than in the low-PVS group. PVS, plasma volume status.
Table 2.
Multivariate analyses of the associations with 365-day all-cause death
| All-cause death | Univariate |
Multivariate |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| PVS | ||||||||||||
| Low | 1.000 | 1.000 | ||||||||||
| High | 1.857 | 1.293-2.667 | 0.001 | 1.642 | 1.049-2.570 | 0.030 | ||||||
| PVS and CONUT score | ||||||||||||
| Low-PVS/low-CONUT | 1.000 | 1.000 | ||||||||||
| High-PVS/low-CONUT | 2.058 | 1.144-3.702 | 0.016 | 1.785 | 0.929-3.340 | 0.082 | ||||||
| Low-PVS/high-CONUT | 2.784 | 1.554-4.986 | 0.001 | 1.422 | 0.767-2.637 | 0.264 | ||||||
| High-PVS/high-CONUT | 3.982 | 2.349-6.750 | < 0.001 | 2.076 | 1.147-3.757 | 0.016 | ||||||
| PVS and PNI | ||||||||||||
| Low-PVS/high-PNI | 1.000 | 1.000 | ||||||||||
| High-PVS/high-PNI | 2.076 | 1.120-3.846 | 0.020 | 1.705 | 0.873-3.332 | 0.118 | ||||||
| Low-PVS/low-PNI | 2.742 | 1.522-4.938 | 0.001 | 1.438 | 0.774-2.674 | 0.251 | ||||||
| High-PVS/low-PNI | 3.814 | 2.238-6.498 | < 0.001 | 2.094 | 1.166-3.761 | 0.013 | ||||||
| Adjusting factors | ||||||||||||
| Status | ||||||||||||
| Age (per 10-y old) | 1.269 | 1.072-1.501 | 0.006 | 1.454 | 1.182-1.790 | < 0.001 | 1.443 | 1.173-1.776 | 0.001 | 1.450 | 1.177-1.787 | < 0.001 |
| Gender (male) | 0.983 | 0.680-1.422 | 0.929 | 1.209 | 0.806-1.814 | 0.359 | 1.185 | 0.787-1.783 | 0.417 | 1.159 | 0.768-1.748 | 0.483 |
| Etiology (ischemia, yes) | 0.816 | 0.567-1.173 | 0.272 | 0.691 | 0.462-1.032 | 0.071 | 0.701 | 0.47-1.047 | 0.083 | 0.706 | 0.471-1.058 | 0.092 |
| Type (readmission, yes) | 1.151 | 0.804-1.646 | 0.442 | 0.717 | 0.481-1.069 | 0.102 | 0.719 | 0.482-1.071 | 0.105 | 0.757 | 0.504-1.137 | 0.179 |
| NYHA (IV, yes) | 1.984 | 1.174-3.353 | 0.011 | 2.201 | 1.276-3.797 | 0.005 | 2.219 | 1.287-3.827 | 0.004 | 2.187 | 1.269-3.769 | 0.005 |
| Medical history (diabetes mellitus, yes) | 1.260 | 0.886-1.792 | 0.198 | 1.465 | 1.008-2.128 | 0.045 | 1.542 | 0.999-2.111 | 0.051 | 1.447 | 0.995-2.104 | 0.053 |
| LVEF (per 10% increase) | 0.953 | 0.860-1.056 | 0.360 | 0.974 | 0.874-1.085 | 0.631 | 0.970 | 0.870-1.081 | 0.58 | 0.974 | 0.874-1.085 | 0.627 |
| Vital signs | ||||||||||||
| SBP (per 10 mm Hg increase) | 0.848 | 0.815-0.882 | < 0.001 | 0.855 | 0.815-0.897 | < 0.001 | 0.859 | 0.817-0.902 | < 0.001 | 0.858 | 0.817-0.902 | < 0.001 |
| Heart rate (per 10 beats/min increase) | 1.004 | 0.961-1.048 | 0.863 | 1.021 | 0.998-1.044 | 0.069 | 1.020 | 0.999-1.042 | 0.068 | 1.020 | 0.999-1.042 | 0.062 |
| Laboratory data | ||||||||||||
| Total bilirubin (per 1 mg/dL increase) | 1.008 | 1.000-1.016 | 0.039 | 1.001 | 0.991-1.010 | 0.914 | 1.000 | 0.990-1.009 | 0.951 | 1.000 | 0.990-1.009 | 0.926 |
| Creatinine (per 0.1 mg/dL increase) | 1.011 | 1.004-1.019 | 0.002 | 1.010 | 1.001-1.019 | 0.032 | 1.010 | 1.001-1.019 | 0.035 | 1.010 | 1.001-1.019 | 0.038 |
| Sodium (per 1.0 mmol/L increase) | 0.946 | 0.919-0.975 | < 0.001 | 0.993 | 0.960-1.026 | 0.661 | 0.996 | 0.963-1.029 | 0.792 | 0.994 | 0.961-1.027 | 0.705 |
| Potassium (per 1.0 mmol/L increase) | 1.537 | 1.265-1.866 | 0.001 | 1.379 | 1.097-1.734 | 0.006 | 1.365 | 1.087-1.715 | 0.007 | 1.363 | 1.087-1.787 | 0.007 |
| Medication | ||||||||||||
| Dobutamine (yes) | 3.027 | 2.122-4.317 | <0.001 | 1.710 | 1.119-2.614 | 0.013 | 1.657 | 1.079-2.543 | 0.021 | 1.657 | 1.080-2.543 | 0.021 |
| ACE-I/ARB (yes) | 0.302 | 0.179-0.510 | <0.001 | 0.594 | 0.343-1.028 | 0.063 | 0.600 | 0.346-1.041 | 0.069 | 0.598 | 0.345-1.037 | 0.067 |
| β-Blocker (yes) | 0.813 | 0.544-1.215 | 0.312 | 0.941 | 0.620-1.429 | 0.775 | 0.944 | 0.622-1.431 | 0.785 | 0.942 | 0.621-1.428 | 0.777 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; CONUT, Controlling Nutritional Status; CRP, C-reactive protein; HR, hazard ratio; LVEF, left ventricular ejection fraction; PNI, prognostic nutritional index; PVS, plasma volume status; SBP, systolic blood pressure.
PVS and nutrition status
Both the low- and high-PVS cohorts in the high-CONUT group had significantly lower systolic blood pressures and heart rates and significantly higher levels of serum blood urea nitrogen and CRP than those in the low-CONUT group. Furthermore, the administration of nitroglycerine was significantly less frequent in patients in the high-CONUT group vs the low-CONUT group. In contrast, the administration of dobutamine was significantly more frequent in the high-CONUT group. Indeed, the duration of ICU stay in patients in the high-CONUT group was significantly longer than in the low-CONUT group (Table 3). This tendency was also observed when using the PNI to assess the nutritional status of patients (Table 4).
Table 3.
Characteristics of patients by the difference in PVS and CONUT
| Low PVS |
P value | High PVS |
P value | |||
|---|---|---|---|---|---|---|
| Low CONUT |
High CONUT |
Low CONUT |
High CONUT |
|||
| (n = 189) | (n = 111) | (n = 141) | (n = 160) | |||
| Status and vital signs | ||||||
| Age (y) | 70 (61-78) | 72 (64-79) | 0.204 | 79 (72-86) | 80 (72-84) | 0.406 |
| Gender (male, %) | 148 (78.3%) | 94 (84.7%) | 0.226 | 61 (43.3%) | 90 (56.3%) | 0.028 |
| Type (readmission, %) | 59 (31.2%) | 33 (29.7%) | 0.897 | 62 (44.0%) | 65 (40.6%) | 0.561 |
| LVEF (%) | 33 (25-47) | 35 (24-51) | 0.939 | 41 (30-52) | 42 (30-57) | 0.584 |
| LVEF ≤ 40% (yes, %) | 119 (63.0%) | 71 (64.0%) | 0.902 | 65 (46.1%) | 77 (48.1%) | 0.729 |
| NYHA (IV, %) | 155 (82.0%) | 84 (75.7%) | 0.234 | 114 (80.9%) | 115 (71.9%) | 0.079 |
| Systolic blood pressure (mm Hg) | 172 (141-199) | 139 (108-166) | < 0.001 | 165 (140-190) | 143 (110-161) | < 0.001 |
| Pulse (beats/min) | 117 (99-136) | 100 (91-120) | < 0.001 | 108 (93-126) | 94 (79-111) | < 0.001 |
| Etiology | ||||||
| Ischemia (yes, %) | 82 (43.4%) | 42 (37.8%) | 0.396 | 61 (43.3%) | 55 (34.4%) | 0.124 |
| Medical history | ||||||
| Hypertension (yes, %) | 142 (75.1%) | 74 (66.7%) | 0.143 | 107 (75.9%) | 129 (80.6%) | 0.329 |
| Diabetes mellitus (yes, %) | 86 (45.5%) | 53 (47.7%) | 0.721 | 77 (54.6%) | 76 (47.5%) | 0.248 |
| Dyslipidemia (yes, %) | 103 (54.5%) | 53 (47.7%) | 0.282 | 72 (51.1%) | 80 (50.0%) | 0.908 |
| Arterial blood gas | ||||||
| pH | 7.31 (7.19-7.41) | 7.40 (7.29-7.45) | < 0.001 | 7.32 (7.20-7.42) | 7.39 (7.32-7.44) | < 0.001 |
| PCO2 (mm Hg) | 45.3 (35.9-57.2) | 35.3 (29.0-46.7) | < 0.001 | 42.8 (34.4-55.6) | 36.4 (32.0-41.8) | < 0.001 |
| PO2 (mm Hg) | 92.6 (69.2-134.0) | 91.1 (75.7-160.0) | 0.233 | 109.0 (74.0-169.0) | 103.0 (69.8-144.0) | 0.065 |
| HCO3- (mmol/L) | 22.3 (19.8-24.5) | 22.0 (19.4-24.4) | 0.550 | 21.3 (18.7-23.7) | 21.3 (18.3-24.2) | 0.758 |
| SaO2 (%) | 96 (92-98) | 97 (94-99) | 0.041 | 97 (92-99) | 97 (94-99) | 0.119 |
| Lactate (mmol/L) | 1.8 (1.2-3.6) | 1.8 (1.2-3.8) | 0.651 | 2.0 (1.2-3.7) | 1.7 (1.0-2.9) | 0.010 |
| Laboratory data | ||||||
| Total bilirubin (μmol/L) | 10.3 (6.8-17.1) | 13.7 (8.6-23.1) | 0.003 | 8.6 (5.1-12.0) | 10.3 (6.8-15.4) | 0.004 |
| Uric acid (μmol/L) | 416 (333-482) | 405 (330-538) | 0.515 | 363 (297-446) | 422 (327-495) | 0.003 |
| Sodium (mmol/L) | 140 (138-142) | 138 (135-141) | < 0.001 | 140 (137-142) | 139 (136-143) | 0.588 |
| Potassium (mmol/L) | 4.2 (4.0-4.6) | 4.3 (3.9-5.0) | 0.227 | 4.3 (3.8-4.8) | 4.3 (3.8-4.7) | 0.708 |
| Hemoglobin (g/L) | 140 (130-153) | 133 (121-142) | <0.001 | 109 (97-122) | 99 (88-110) | < 0.001 |
| BUN (μmol/L) | 7.5 (5.9-10.3) | 9.8 (6.6-15.0) | 0.001 | 9.8 (6.5-14.9) | 13.8 (8.6-19.2) | < 0.001 |
| Creatinine (μmol/L) | 95.5 (73.4-126.4) | 118.5 (3.1-169.3) | 0.003 | 106.1 (73.4-188.3) | 148.1 (89.1-256.4) | 0.008 |
| CRP (μg/L) | 5200 (1800-16,800) | 17,800 (4550-59,850) | < 0.001 | 3700 (1100-12,100) | 24,800 (5050-77,700) | < 0.001 |
| BNP (pmol/L) | 21.5 (10.4-100.9) | 36.3 (16.6-979.3) | 0.008 | 41.2 (20.2-121.1) | 40.8 (19.6-136.2) | 0.684 |
| Nutritional status | ||||||
| PNI | 48.3 (44.8-57.5) | 37.8 (34.7-40.2) | < 0.001 | 46.1 (42.6-53.1) | 35.5 (31.3-39.0) | < 0.001 |
| CONUT score | 1 (0-2) | 5 (4-6) | < 0.001 | 2 (1-3) | 6 (5-7) | < 0.001 |
| Albumin (g/L) | 38 (35-40) | 33 (30-35) | < 0.001 | 36 (35-38) | 31 (28-33) | < 0.001 |
| Lymphocyte count (/μL) | 2194 (1524-3880) | 765 (542-1211) | < 0.001 | 1843 (1400-3136) | 714 (487-1036) | < 0.001 |
| Total cholesterol (mmol/L) | 4.73 (4.03-5.61) | 3.65 (3.30-4.32) | < 0.001 | 4.65 (4.03-5.20) | 3.61 (3.10-4.39) | < 0.001 |
| Medication (cases) during ICU | ||||||
| Furosemide (yes, %) | 171 (90.5%) | 98 (88.3%) | 0.560 | 127 (90.1%) | 145 (90.6%) | 1.000 |
| Nitroglycerin (yes, %) | 94 (49.7%) | 28 (25.2%) | < 0.001 | 76 (53.9%) | 52 (32.5%) | < 0.001 |
| Nicorandil (yes, %) | 29 (15.4%) | 14 (12.6%) | 0.610 | 20 (14.2%) | 32 (20.0%) | 0.222 |
| Carperitide (yes, %) | 69 (36.5%) | 43 (38.7%) | 0.712 | 49 (34.8%) | 60 (37.5%) | 0.633 |
| Dopamine (yes, %) | 10 (5.3%) | 9 (8.1%) | 0.337 | 5 (3.5%) | 14 (8.8%) | 0.095 |
| Dobutamine (yes, %) | 32 (16.9%) | 36 (32.4%) | 0.003 | 17 (12.1%) | 47 (29.4%) | < 0.001 |
| ACE-I/ARB (yes, %) | 76 (40.2%) | 32 (28.8%) | 0.061 | 41 (29.1%) | 33 (20.6%) | 0.107 |
| β-Blocker (yes, %) | 69 (36.5%) | 32 (28.8%) | 0.206 | 38 (27.0%) | 32 (20.0%) | 0.173 |
| Spironolactone (yes, %) | 85 (45.0%) | 46 (41.4%) | 0.630 | 42 (29.8%) | 42 (26.3%) | 0.521 |
| Statin (yes, %) | 63 (33.3%) | 33 (29.7%) | 0.608 | 45 (31.9%) | 41 (25.6%) | 0.251 |
| Outcome | ||||||
| ICU hospitalization (d) | 3 (3-5) | 5 (3-10) | < 0.001 | 3 (2-4) | 4 (3-7) | < 0.001 |
| Total hospitalization (d) | 19 (14-31) | 32 (20-47) | < 0.001 | 23 (16-38) | 28 (18-47) | 0.151 |
| In-hospital mortality (yes, %) | 9 (4.8%) | 19 (17.1%) | 0.001 | 13 (9.2%) | 30 (18.8%) | 0.021 |
All numerical data are expressed as the median (25%-75% interquartile range). P values between the low-CONUT and high-CONUT groups were determined using the Mann–Whitney U test or chi-square test.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CONUT, Controlling Nutritional Status; CRP, C-reactive protein; ICU, intensive care unit; NYHA, New York Heart Association; PNI, prognostic nutritional index; PVS, plasma volume status; LVEF, left ventricular ejection fraction.
Table 4.
Characteristics of the patients by the difference in PVS and PNI
| Low PVS |
P value | High PVS |
P value | |||
|---|---|---|---|---|---|---|
| High PNI |
Low PNI |
High PNI |
Low PNI |
|||
| (n = 180) | (n = 120) | (n = 121) | (n = 180) | |||
| Status and vital signs | ||||||
| Age (y) | 70 (61-78) | 72 (64-79) | 0.166 | 79 (71-85) | 80 (72-84) | 0.127 |
| Gender (male, %) | 140 (77.8%) | 102 (85.0%) | 0.137 | 54 (44.6%) | 97 (53.9%) | 0.751 |
| Type (readmission, %) | 61 (33.9%) | 31 (25.8%) | 0.160 | 57 (47.1%) | 70 (38.9%) | 0.190 |
| LVEF (%) | 33 (25-47) | 36 (22-50) | 0.986 | 41 (30-57) | 42 (30-53) | 0.550 |
| LVEF ≤ 40% (yes, %) | 114 (63.3%) | 76 (63.3%) | 1.000 | 58 (47.9%) | 84 (46.7%) | 0.906 |
| NYHA (IV, %) | 146 (81.1%) | 93 (77.5%) | 0.467 | 99 (81.8%) | 130 (72.2%) | 0.073 |
| Systolic blood pressure (mm Hg) | 172 (146-200) | 140 (108-168) | < 0.001 | 162 (139-190) | 146 (113-167) | < 0.001 |
| Pulse (beats/min) | 119 (100-138) | 100 (86-120) | < 0.001 | 108 (90-126) | 98 (81-112) | 0.002 |
| Etiology | ||||||
| Ischemia (yes, %) | 77 (42.8%) | 47 (39.2%) | 0.552 | 49 (40.5%) | 67 (37.2%) | 0.629 |
| Medical history | ||||||
| Hypertension (yes, %) | 134 (74.4%) | 82 (68.3%) | 0.294 | 98 (81.0%) | 133 (76.7%) | 0.395 |
| Diabetes mellitus (yes, %) | 82 (45.6%) | 57 (47.5%) | 0.813 | 67 (55.4%) | 86 (47.8%) | 0.240 |
| Dyslipidemia (yes, %) | 103 (57.2%) | 53 (44.2%) | 0.034 | 60 (49.6%) | 92 (51.1%) | 0.815 |
| Arterial blood gas | ||||||
| pH | 7.30 (7.19-7.40) | 7.40 (7.30-7.45) | < 0.001 | 7.30 (7.18-7.40) | 7.39 (7.31-7.44) | < 0.001 |
| PCO2 (mm Hg) | 46.7 (36.7-57.7) | 35.0 (29.1-43.7) | < 0.001 | 46.3 (35.7-60.2) | 35.5 (31.1-41.6) | < 0.001 |
| PO2 (mm Hg) | 90.3 (68.2-134.0) | 94.7 (75.4-145.0) | 0.140 | 109.5 (76.8-156.0) | 103.0 (69.4-147.5) | 0.091 |
| HCO3- (mmol/L) | 22.4 (20.0-24.6) | 21.8 (18.9-24.2) | 0.112 | 21.3 (19.3-23.6) | 21.3 (18.1-24.3) | 0.897 |
| SaO2 (%) | 96 (92-98) | 97 (94-99) | 0.008 | 96 (92-98) | 98 (93-99) | 0.085 |
| Lactate (mmol/L) | 1.8 (1.2-3.7) | 1.9 (1.2-3.6) | 0.682 | 2.1 (1.2-4.2) | 1.7 (1.0-2.9) | 0.006 |
| Laboratory data | ||||||
| Total bilirubin (mg/dL) | 10.3 (6.8-17.4) | 12.0 (8.6-21.0) | 0.007 | 8.6 (5.1-12.0) | 10.3 (6.8-15.4) | 0.014 |
| Uric acid (mg/dL) | 413 (333-470) | 416 (332-537) | 0.117 | 357 (297-446) | 416 (326-494) | 0.005 |
| Sodium (mmol/L) | 140 (138-142) | 139 (135-141) | < 0.001 | 140 (138-142) | 139 (136-143) | 0.530 |
| Potassium (mmol/L) | 4.2 (4.0-4.6) | 4.3 (3.9-5.0) | 0.176 | 4.3 (3.9-4.7) | 4.3 (3.8-4.9) | 0.932 |
| Hemoglobin (g/dL) | 140 (129-153) | 133 (123-142) | < 0.001 | 111 (100-122) | 98 (88-111) | < 0.001 |
| BUN (mmol/L) | 7.4 (5.8-10.1) | 9.7 (6.9-14.6) | < 0.001 | 9.0 (6.3-14.1) | 13.7 (9.1-19.5) | < 0.001 |
| Creatinine (g/dL) | 95.0 (73.4-126.4) | 115.4 (86.0-163.5) | 0.001 | 100.8 (72.5-168.0) | 152.1 (89.3-256.4) | 0.001 |
| CRP (mg/dL) | 4500 (1775-14,425) | 21,000 (5375-61,725) | < 0.001 | 2600 (1000-8500) | 24,400 (5325-68,625) | < 0.001 |
| BNP (pg/mL) | 21.7 (10.6-84.1) | 37.5 (14.8-1140.7) | 0.017 | 35.6 (17.9-75.1) | 47.0 (21.0-147.5) | 0.072 |
| Nutritional status | ||||||
| PNI | 49.2 (45.7-58.6) | 38.0 (35.0-39.8) | < 0.001 | 48.6 (45.1-55.2) | 36.3 (32.0-39.3) | < 0.001 |
| CONUT score | 1 (0-2) | 5 (4-6) | < 0.001 | 2 (0-3) | 6 (4-7) | < 0.001 |
| Albumin (g/L) | 38 (36-40) | 33 (30-35) | < 0.001 | 37 (35-39) | 32 (29-34) | < 0.001 |
| Lymphocyte count (/μL) | 2339 (1613-4130) | 814 (542-1248) | < 0.001 | 2309 (1658-3832) | 741 (490-1117) | < 0.001 |
| Total cholesterol (mmol/L) | 4.59 (3.88-5.54) | 3.84 (3.36-4.69) | < 0.001 | 4.55 (3.78-5.12) | 3.98 (3.31-4.73) | < 0.001 |
| Medication (cases) during ICU | ||||||
| Flurosemide (yes, %) | 162 (90.0%) | 107 (89.2%) | 0.848 | 111 (91.7%) | 161 (89.4%) | 0.556 |
| Nitroglycerin (yes, %) | 90 (50.0%) | 32 (26.7%) | < 0.001 | 66 (54.5%) | 62 (34.4%) | 0.001 |
| Nicorandil (yes, %) | 27 (15.0%) | 16 (13.3%) | 0.739 | 18 (14.9%) | 34 (18.9%) | 0.438 |
| Carperitide (yes, %) | 63 (35.0%) | 49 (40.8%) | 0.331 | 42 (34.7%) | 67 (37.2%) | 0.714 |
| Dopamine (yes, %) | 10 (5.6%) | 9 (7.5%) | 0.629 | 4 (3.3%) | 15 (8.3%) | 0.093 |
| Dobutamine (yes, %) | 29 (16.1%) | 39 (32.5%) | 0.001 | 17 (14.0%) | 47 (26.1%) | 0.014 |
| ACE-I/ARB (yes, %) | 72 (40.0%) | 36 (30.0%) | 0.086 | 34 (28.1%) | 40 (22.2%) | 0.276 |
| β-Blocker (yes, %) | 68 (37.8%) | 33 (27.5%) | 0.081 | 30 (24.8%) | 40 (22.2%) | 0.677 |
| Spironolactone (yes, %) | 75 (41.7%) | 56 (46.7%) | 0.408 | 39 (32.2%) | 45 (25.0%) | 0.191 |
| Statin (yes, %) | 62 (34.4%) | 34 (28.3%) | 0.312 | 38 (31.4%) | 48 (26.7%) | 0.435 |
| Outcome | ||||||
| ICU hospitalization (d) | 3 (3-5) | 5 (3-10) | < 0.001 | 3 (2-4) | 4 (3-6) | 0.001 |
| Total hospitalization (d) | 19 (15-30) | 32 (19-47) | < 0.001 | 22 (16-37) | 29 (17-47) | 0.060 |
| In-hospital mortality (yes, %) | 8 (4.4%) | 20 (16.7%) | < 0.001 | 12 (9.9%) | 31 (17.2%) | 0.093 |
P values between the high-PNI and low-PNI groups were determined using the Mann–Whitney U test or chi-square test. All numerical data are expressed as the median (25%-75% interquartile range).
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CONUT, Controlling Nutritional Status; CRP, C-reactive protein; ICU, intensive care unit; NYHA, New York Heart Association; PNI, prognostic nutritional index; LVEF, left ventricular ejection fraction; PVS, plasma volume status.
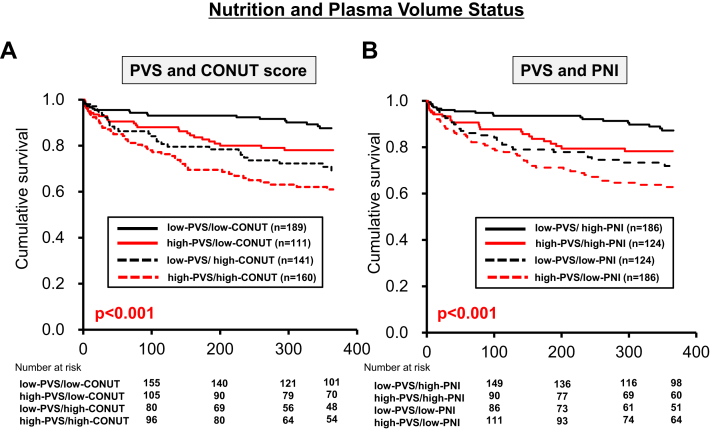
The Kaplan–Meier curves for the low- or high-PVS/CONUT and low- or high-PVS/PNI groups are shown in Figure 3. The prognosis in both the high- and low-PVS groups, including all-cause death within 365 days, was significantly poorer in patients with a high CONUT score than in those with a low CONUT score (Fig. 3A). Similar prognostic results were observed for patients with a low PNI vs those with a high PNI (Fig. 3B). The multivariate Cox proportional hazards regression model showed that high PVS/high CONUT was an independent predictor of 365-day mortality (HR, 2.076; 95% CI, 1.147-3.757; P = 0.016) (Table 2). A similar result was obtained for the PNI; only high PVS/low PNI was an independent predictor of 365-day mortality (HR, 2.094; 95% CI, 1.166-3.761; P = 0.013) (Table 2). These results suggest that the evaluation of PVS alone is insufficient to predict mortality caused by AHF. Evaluation of both the nutritional status and PVS is required for the accurate prediction of mortality in patients with severely decompensated AHF.
Figure 3.
Kaplan–Meier survival curves for PVS and nutrition status. (A) Kaplan–Meier survival curves showed that the prognosis, including all-cause death, was significantly poorer in the high-CONUT/high-PVS group than in the low-CONUT/high-PVS group. (B) Kaplan–Meier survival curves showed that the prognosis, including all-cause death, was significantly poorer in the low-PNI/high-PVS group than in the high-PNI/high-PVS group. CONUT, Controlling Nutritional Status; PNI, prognostic nutritional index; PVS, plasma volume status.
Discussion
The present study revealed that a high PVS was clearly associated with an adverse outcome. Furthermore, a high PVS combined with malnutrition led to an adverse outcome. Therefore, a high PVS and malnutrition were essential factors in the AHF cohort. The prognostic impact of each factor has been demonstrated in previous studies.6, 7, 8, 9,12, 13, 14, 15, 16 Therefore, the novelty of the present study vs previous investigations lies in the simultaneous evaluation of these factors. These findings underscore the importance of a prompt evaluation to determine both the general congestion and the nutritional status of patients with AHF, as well as the need for early intervention in these patients.
Tools for evaluating PV and AHF
In 2015, the importance of a noninvasive and simple estimation of PV in patients with HF was recognized, and formulae for predicting future cardiovascular events and mortality have been proposed.9,21 Duarte et al.21 used the Strauss formula (ie, changes in the concentrations of hemoglobin and hematocrit) to estimate the PV. They concluded that a higher instantaneous estimated PVS was significantly associated with a poorer outcome in patients with left ventricular systolic dysfunction after acute myocardial infarction.21 Kobayashi et al.8 also determined the prognostic impact of PVS during the discharge of patients with AHF using the Strauss formula.22 Yoshihisa et al.6 showed that high PVS estimated using the Hakim formula (ie, using hematocrit and weight, with the same formula as that used in the present study) at admission was associated with a poor outcome in patients with AHF. Furthermore, estimating PVS in dyspneic patients in the emergency department using the Strauss formula has been shown to have diagnostic value for AHF.7 Thus, immediate evaluation of the PV in patients with AHF is required to predict prognosis. The optimal methodology for evaluating the PV remains debatable; in the present study, the formula incorporating weight was used. Weight is an essential factor when evaluating the general volume status. The results of the present study are consistent with those reported in previous studies.
The strategy for estimating the PV has differed among studies. Although the measurement of congestion using pulmonary artery catheterization,23 echocardiography,24 and volume biomarkers25 is traditionally reported, an immediate, simple, and noninvasive methodology may be required for an early intervention of volume expansion. The Strauss formula, which uses the levels of hemoglobin and hematocrit, may be affected by the anemia status. Anemia at admission or within 3 days after admission is an independent risk factor for future adverse events in patients with AHF.26,27 Therefore, the Hakim formula was selected to estimate the PV.
Malnutrition and PVS in AHF
Malnutrition is a common complication in patients with chronic HF. Previous reports have attributed malnutrition to a low nutritional intake or malabsorption due to intestinal edema, anorexia, liver dysfunction, or cytokine-induced hypercatabolism.28 Patients develop enteral protein loss, and the total daily energy expenditure shifts to a catabolic state. Therefore, malnutrition leads to cachexia and is occasionally complicated by inflammation. Inflammation itself is an independent risk factor for AHF,16 and cachexia is associated with an adverse outcome.13 Under these circumstances, weight loss develops, leading to marked worsening of prognosis.29 Both malnutrition and inflammation are major factors affecting volume expansion in patients with AHF. Indeed, the degree of volume expansion is exacerbated in patients with an extremely poor nutritional status. Because these patients tend to develop adverse outcomes, an immediate procedural intervention must be considered.
Decongestion is the mainstay for the treatment of patients with AHF requiring intensive care. Diuretics have been proposed as the gold standard for treating patients with volume overload, and this strategy must be immediately applied in the emergency department.2 Tolvaptan was suggested as a new therapeutic option in Japan in 2010, and the immediate administration of tolvaptan was recommended in the acute phase of AHF.30 Evidence also supports the use of other traditional diuretics (ie, loop diuretics, spironolactone, and thiazide diuretics) in the acute phase. Continuous renal replacement therapy or the extracorporeal ultrafiltration method may be used for patients with acute kidney injury or end-stage chronic kidney disease. Several days are required for the treatment of decongestion in patients with high PVS. Therefore, treating physicians may hesitate to initiate treatments for myocardial protection (eg, angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers, β-blockers, and spironolactone) in intensive care because of the remaining congestion from the acute phase. Complete decongestion is a priority for treatment in intensive care. These medical differences may influence the prognosis in each group. Therefore, we added the medication as an adjusting factor in the multivariate model. As mentioned earlier, numerous procedures are available for managing decongestion. However, on the basis of the results of the present study, PV overload and malnutrition must be treated. Therefore, combination therapy may be required for such patients.
Medical intervention for malnutrition has been reported in patients with chronic HF with complications from inflammation, cachexia, and wasting.31,32 Malnutrition is related to deprivation of macronutrients (eg, protein and energy) and micronutrients (eg, amino acids, vitamins, and minerals). Supplementation of essential amino acid has been suggested to improve the nutritional and metabolic status in patients with HF-related wasting syndrome under optimal medical treatment and adequate protein-energy intake.33 However, thus far, immediate and aggressive intervention to manage malnutrition during the acute phase of AHF has not been reported. The nutritional status is mainly evaluated using the concentration of serum albumin during the acute phase of AHF, because this can be easily and inexpensively obtained through a routine laboratory test. Currently, it is unclear whether a single parameter, such as serum albumin, can be used to assess the nutritional status. However, the presence of hypoalbuminemia may be useful for identifying extremely sick patients in the AHF cohort. However, malnutrition is only one factor inducing hypoalbuminemia in patients with AHF. Furthermore, whether or not nutritional and anti-inflammatory interventions can be relied on, at least in part, to increase the concentration of serum albumin in patients with HF is unclear.31 There is no evidence to suggest that administration of human albumin increases the concentration of serum albumin in patients with HF. Although several randomized controlled trials and meta-analyses have been conducted in critically ill patients in the ICU, few provided positive data.34, 35, 36 The 1-month mortality rate among patients who received albumin was similar to that reported for patients who received saline.34 However, when limiting the discussion to those who achieved a serum albumin concentration > 3 g/dL, the administration of human albumin was able to reduce the rate of adverse outcomes in critically ill patients.37 Further research is required to determine the benefits of targeted nutritional and anti-inflammatory interventions, as well as the administration of albumin, for the survival of select patients with HF and hypoalbuminemia. Valentova et al.38 reported that patients with cardiac cachexia display a more pronounced degree of right ventricular failure and hypoalbuminemia compared with noncachectic patients of a similar LVEF and NYHA class. Patients with high PVS are occasionally categorized as having biventricular AHF; thus, a majority of these patients had complicated right HF. Therefore, a high PVS coupled with malnutrition may complicate right HF. In a right ventricular volume overload model, administration of S-nitroso human serum albumin improved right ventricular-arterial coupling and reduced oxidative stress.39 This positive intervention may lead to a better outcome in patients with AHF with high PVS complicated by malnutrition via improvement of right ventricular function.
The benefits of early nutritional intervention by enteral tube feeding vs parenteral central venous catheter and the best methodology for enteral intervention are current topics in intensive care.40,41 Strategies for the amelioration of the nutritional status and volume status must be considered during the acute phase of AHF to improve the poor prognosis of patients with malnutrition and volume expansion.
Study limitations
The present study has several limitations. First, the study population was limited to patients admitted to the ICU. Thus, patients with AHF admitted to the general wards were excluded from this study. This exclusion may reduce the generalizability of the present results. In our institute, the patients were treated by cardiologists in a “closed ICU.” Thus, the majority of severely decompensated patients with AHF were admitted to the ICU. However, clear criteria regarding the dose of high-flow oxygen, inotropes, and diuretics were not proposed. Moreover, the admission criteria may have differed annually. The physician ultimately decided where each patient should be admitted in the hospital (ie, ICU or general ward), and patient bias may have affected this decision. Second, the study was performed at a single center and was not a prospective, randomized, controlled trial. Therefore, it is possible that unmeasured variables affected the results. Furthermore, the difficulty in standardizing care for each patient may have influenced the major findings of this study. Third, body weight before admission was adopted as the compensated dry weight. However, this may not accurately represent the patient’s dry weight and may even be more unreliable than using weight at admission. However, the most important issue was to avoid using the weight during volume overload. Therefore, the historical weight was used to represent the compensated weight. Fourth, although the percentage of patients receiving statin therapy during the ICU stay was revealed, the accurate percentage on admission was not determined. It may be important to know the actual number of patients receiving lipid-lowering therapy and whether this number affects the calculations related to nutrition. Fifth, the majority of patients in the present study had de novo HF (n = 382, 63.6%), which renders it difficult to reconcile cardiac cachexia and other findings. Finally, the prognoses of patients being followed up at other institutes were determined through unstructured telephone interviews. Therefore, HF events may have been missed.
Conclusions
PV expansion on admission was shown to be associated with adverse outcomes in patients with AHF; however, this value alone was insufficient for an accurate prognosis. The simultaneous evaluation of nutrition status and PVS is essential to predict the outcome of patients with severely decompensated AHF.
Acknowledgements
The authors thank the staff of the ICU and the Medical Records Office at Chiba Hokusoh Hospital, Nippon Medical School, for collecting the medical data.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The Research Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital approved the study protocol. Because of the retrospective design of the study, written informed consent was waived, in accordance with the guidance provided by the Ethics Committee. Research Ethics Committee information; Reference number; 543-1.
See page 314 for disclosure information.
References
- 1.Mebazaa A., Gheorghiade M., Pina I.L. Practical recommendations for prehospital and early in-hospital management of patients presenting with acute heart failure syndromes. Crit Care Med. 2008;36:S129–S139. doi: 10.1097/01.CCM.0000296274.51933.4C. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz J.A., O'Meara E., McDonald M.A. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Cotter G., Metra M., Milo-Cotter O., Dittrich H.C., Gheorghiade M. Fluid overload in acute heart failure--re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Dupont M., Mullens W., Tang W.H. Impact of systemic venous congestion in heart failure. Curr Heart Fail Rep. 2011;8:233–241. doi: 10.1007/s11897-011-0071-7. [DOI] [PubMed] [Google Scholar]
- 6.Yoshihisa A., Abe S., Sato Y. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2018;7:330–338. doi: 10.1177/2048872617690889. [DOI] [PubMed] [Google Scholar]
- 7.Chouihed T., Rossignol P., Bassand A. Diagnostic and prognostic value of plasma volume status at emergency department admission in dyspneic patients: results from the PARADISE cohort. Clin Res Cardiol. 2019;108:563–573. doi: 10.1007/s00392-018-1388-y. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M., Rossignol P., Ferreira J.P. Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin Res Cardiol. 2019;108:549–561. doi: 10.1007/s00392-018-1385-1. [DOI] [PubMed] [Google Scholar]
- 9.Ling H.Z., Flint J., Damgaard M. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail. 2015;17:35–43. doi: 10.1002/ejhf.193. [DOI] [PubMed] [Google Scholar]
- 10.Lin H., Zhang H., Lin Z. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev. 2016;21:549–565. doi: 10.1007/s10741-016-9540-0. [DOI] [PubMed] [Google Scholar]
- 11.Sargento L., Longo S., Lousada N., dos Reis R.P. The importance of assessing nutritional status in elderly patients with heart failure. Curr Heart Fail Rep. 2014;11:220–226. doi: 10.1007/s11897-014-0189-5. [DOI] [PubMed] [Google Scholar]
- 12.Uthamalingam S., Kandala J., Daley M. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J. 2010;160:1149–1155. doi: 10.1016/j.ahj.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Iwakami N., Nagai T., Furukawa T.A. Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int J Cardiol. 2017;230:529–536. doi: 10.1016/j.ijcard.2016.12.064. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y.L., Sung S.H., Cheng H.M. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arques S. [Serum albumin and heart failure: recent advances on a new paradigm] Ann Cardiol Angiol. 2011;60:272–278. doi: 10.1016/j.ancard.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Bonilla-Palomas J.L., Gamez-Lopez A.L., Moreno-Conde M. Hypoalbuminemia in acute heart failure patients: causes and its impact on hospital and long-term mortality. J Card Fail. 2014;20:350–358. doi: 10.1016/j.cardfail.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Shirakabe A., Hata N., Kobayashi N. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Heart Vessel. 2018;33:134–144. doi: 10.1007/s00380-017-1034-z. [DOI] [PubMed] [Google Scholar]
- 18.Ben Morrison T., Jared Bunch T., Gersh B.J. Pathophysiology of concomitant atrial fibrillation and heart failure: implications for management. Nat Clin Pract Cardiovasc Med. 2009;6:46–56. doi: 10.1038/ncpcardio1414. [DOI] [PubMed] [Google Scholar]
- 19.Onodera T., Goseki N., Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients] Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 20.Ignacio de Ulibarri J., Gonzalez-Madrono A., de Villar N.G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 21.Duarte K., Monnez J.M., Albuisson E. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail. 2015;3:886–893. doi: 10.1016/j.jchf.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Strauss M.B., Davis R.K., Rosenbaum J.D., Rossmeisl E.C. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest. 1951;30:862–868. doi: 10.1172/JCI102501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binanay C., Califf R.M., Hasselblad V. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 24.Santarelli S., Russo V., Lalle I. Prognostic value of decreased peripheral congestion detected by Bioelectrical Impedance Vector Analysis (BIVA) in patients hospitalized for acute heart failure: BIVA prognostic value in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2017;6:339–347. doi: 10.1177/2048872616641281. [DOI] [PubMed] [Google Scholar]
- 25.Masson S., Latini R., Anand I.S. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52:997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 26.Felker G.M., Gattis W.A., Leimberger J.D. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92:625–628. doi: 10.1016/s0002-9149(03)00740-9. [DOI] [PubMed] [Google Scholar]
- 27.Shirakabe A., Hata N., Kobayashi N. Prognostic benefit of maintaining the hemoglobin level during the acute phase in patients with severely decompensated acute heart failure. Heart Vessel. 2018;33:264–278. doi: 10.1007/s00380-017-1057-5. [DOI] [PubMed] [Google Scholar]
- 28.Krack A., Sharma R., Figulla H.R., Anker S.D. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26:2368–2374. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita M., Shirakabe A., Hata N. Association between the body mass index and the clinical findings in patients with acute heart failure: evaluation of the obesity paradox in patients with severely decompensated acute heart failure. Heart Vessel. 2017;32:600–608. doi: 10.1007/s00380-016-0908-9. [DOI] [PubMed] [Google Scholar]
- 30.Shirakabe A., Hata N., Yamamoto M. Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circ J. 2014;78:911–921. doi: 10.1253/circj.cj-13-1255. [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K., Anker S.D., Horwich T.B., Fonarow G.C. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol. 2008;101:89E–103E. doi: 10.1016/j.amjcard.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arques S., Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail. 2011;17:451–458. doi: 10.1016/j.cardfail.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Aquilani R., Viglio S., Iadarola P. Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol. 2008;101 doi: 10.1016/j.amjcard.2008.03.008. 104E-10E. [DOI] [PubMed] [Google Scholar]
- 34.Finfer S., Bellomo R., Boyce N. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 35.Liberati A., Moja L., Moschetti I., Gensini G.F., Gusinu R. Human albumin solution for resuscitation and volume expansion in critically ill patients. Intern Emerg Med. 2006;1:243–245. doi: 10.1007/BF02934748. [DOI] [PubMed] [Google Scholar]
- 36.Wilkes M.M., Navickis R.J. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135:149–164. doi: 10.7326/0003-4819-135-3-200108070-00007. [DOI] [PubMed] [Google Scholar]
- 37.Vincent J.L., Dubois M.J., Navickis R.J., Wilkes M.M. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319–334. doi: 10.1097/01.SLA.0000055547.93484.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valentova M., von Haehling S., Krause C. Cardiac cachexia is associated with right ventricular failure and liver dysfunction. Int J Cardiol. 2013;169:219–224. doi: 10.1016/j.ijcard.2013.08.134. [DOI] [PubMed] [Google Scholar]
- 39.Rungatscher A., Hallstrom S., Linardi D. S-nitroso human serum albumin attenuates pulmonary hypertension, improves right ventricular-arterial coupling, and reduces oxidative stress in a chronic right ventricle volume overload model. J Heart Lung Transplant. 2015;34:479–488. doi: 10.1016/j.healun.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 40.Chapman M., Peake S.L., Bellomo R. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379:1823–1834. doi: 10.1056/NEJMoa1811687. [DOI] [PubMed] [Google Scholar]
- 41.Reignier J., Boisrame-Helms J., Brisard L. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2) Lancet. 2018;391:133–143. doi: 10.1016/S0140-6736(17)32146-3. [DOI] [PubMed] [Google Scholar]