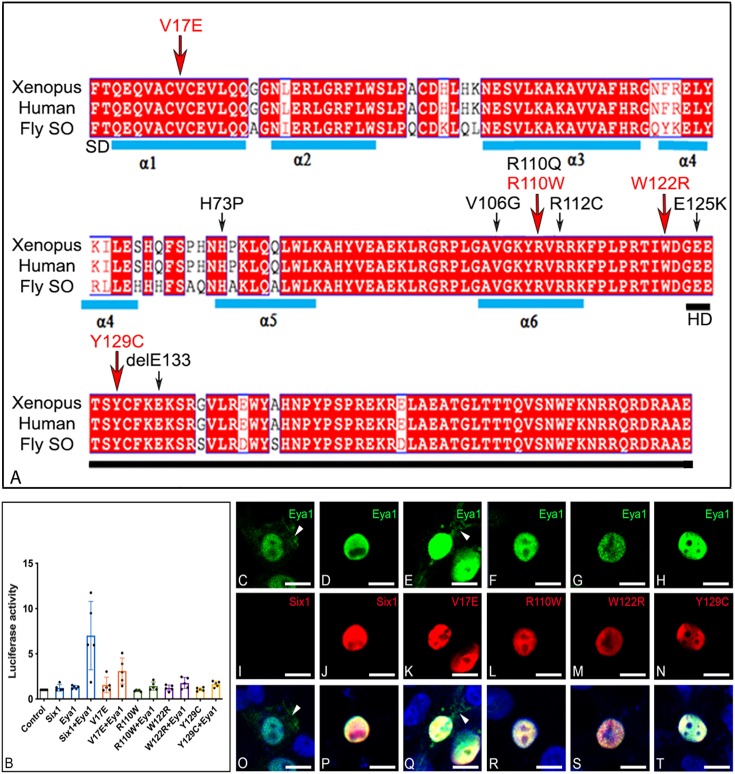
Fig. 1.
BOS/BOR mutations and their transcriptional effects. (A) Amino acid alignment of the N-terminal region of Xenopus laevis Six1, human SIX1 and Drosophila Sine oculis (SO) shows a high level of identity across species; human and frog are 100% identical in this region; differences from fly are in white. The sequence shown begins with the six domain (SD), which contains six α-helices (blue bars), and ends with the homeodomain (HD, black bar). Amino acid substitutions/deletions that have been reported in human BOS/BOR patients are indicated with arrows; red arrows indicate the four mutations that were examined in this study. (B) Expression of Six1+Eya1 caused a significant ∼7-fold increase in luciferase activity when compared to activity of control vector (P<0.0001), Six1WT alone (P<0.0001) or Eya1 alone (P<0.0001). Each mutant plus Eya1 failed to significantly induce luciferase activity relative to control (V17E, P=0.27122; R110W, P=0.99999; W122R, P=0.99764; Y129C, P=0.99947) or in the absence of Eya1 (V17E, P=0.99988; R110W, P=0.99999; W122R, P=0.99999; Y129C, P=0.99999). Experiments were repeated five independent times and subjected to a one-way ANOVA with Tukey post hoc multiple comparisons test. Bars=mean±s.d. (C,I,O). HEK293T cells transfected with only Myc-Eya1 show both cytoplasmic (arrowheads) and nuclear localization. (D,J,P) Cells co-transfected with both Six1WT-FLAG and Myc-Eya1 show nuclear colocalization of both proteins. (E,K,Q) Those transfected with both V17E-FLAG and Myc-Eya1 showed nuclear colocalization and some cytoplasmic Eya1 (arrowheads). Those transfected with (F,L,R) R110W-FLAG and Myc-Eya1, (G,M,S) W122R-FLAG and Myc-Eya1, or (H,N,T) Y129C-FLAG and Myc-Eya1 showed exclusive nuclear colocalization. Scale bars: 10 µm.