Objectives:
Septic shock is often complicated by severe metabolic acidosis, for which renal replacement therapy may be considered. However, little is known about the use of intermittent hemodialysis to manage this condition. The aim of this study was to compare physiologic and biochemical variables and vasopressor requirements before and after intermittent hemodialysis among patients who received intermittent hemodialysis to manage metabolic acidosis during resuscitation of septic shock.
Design:
This retrospective, cross-sectional study was conducted between April 2014 and September 2015.
Settings:
The ICU of a non-university-affiliated teaching hospital.
Patients:
Patients who were admitted to the ICU with septic shock and underwent intermittent hemodialysis to manage metabolic acidosis within 48 hours after the diagnosis of septic shock.
Measurements and Main Results:
The main outcomes were mean arterial pressure, minute ventilator volume, norepinephrine requirement, bicarbonate and pH before and after intermittent hemodialysis. Of 1,190 patients screened, 34 were included, and 33 accomplished a planned session of intermittent hemodialysis. After intermittent hemodialysis, an increased mean arterial pressure (+9.0 mm Hg; 95% CI, 6–13; p < 0.001), decreased minute ventilatory volume (–2.0 L/min; 95% CI, –3.3 to 0.8; p = 0.002), decreased norepinephrine requirement (–0.07 µg/kg/min; 95% CI, –0.12 to –0.02; p = 0.009), increased bicarbonate level (+7.2 mmol/L; 95% CI, 6.1–8.3; p < 0.001), and increased pH (+0.17; 95% CI, 0.13–0.21; p < 0.001) were observed in comparison to those before intermittent hemodialysis.
Conclusions:
In conclusion, intermittent hemodialysis appeared to be feasible and to stabilize hemodynamic and respiratory conditions in patients with septic shock complicated by metabolic acidosis during resuscitation.
Keywords: acute kidney injury, hemodialysis, intensive care, metabolic acidosis, renal replacement therapy, septic shock
Septic shock is often complicated by severe metabolic acidosis. Therapeutic intervention to correct metabolic acidosis may be considered when hemodynamic and respiratory deterioration rapidly progress (1, 2). In general, continuous renal replacement therapy (CRRT) is the preferred mode of renal replacement therapy (RRT) in hemodynamically unstable critically ill patients with regard to hemodynamic stability (1, 3, 4). However, intermittent hemodialysis (IHD) may have potential advantages for those requiring RRT to manage clinically significant metabolic acidosis during resuscitation of septic shock in that IHD more rapidly removes low molecular weight solutes than CRRT (4). Currently, little is known about the use of IHD to manage this condition (5).
IHD rather than CRRT may be employed as a mode of RRT even for hemodynamically unstable patients for various reasons such as cost and limited access to CRRT. For example, in our ICU, 24/7 CRRT service was not available. In addition, CRRT is performed with very low intensity (12–16 mL/kg/hr) in Japan because of a daily maximum dose limitation of CRRT solution products covered by public health insurance (6). Although CRRT with an intensity of greater than or equal to 35 mL/kg/hr has been demonstrated to have no benefit as compared with that with an intensity of 20–25 mL/kg/hr (7–9), one may be concerned about providing CRRT with a very low intensity in such conditions as rapid solute removal is thought to be ideal.
The aim of this study was to compare respiratory physiology and acid-base biochemistry and vasopressor requirements before and after IHD among patients who had metabolic acidosis during resuscitation of septic shock.
MATERIALS AND METHODS
Study Design and Setting
This retrospective, cross-sectional study was conducted at an ICU in Kameda Medical Center between April 2014 and September 2015. Kameda Medical Center is a non-university-affiliated teaching hospital with 917 beds. The ICU was a medical-surgical ICU with a closed-ICU policy and approximately 1,000 annual admissions.
Ethics
The institutional review boards of the hospital approved the study protocol (approval number: 15-071). Requirement to obtain informed consent was waived by them.
Patients
Septic shock patients who received IHD in the ICU for the management of metabolic acidosis within 48 hours after the diagnosis of septic shock were included. Septic shock was defined based on the 2001 International Sepsis Definitions (10). Decision to perform IHD for the correction of metabolic acidosis was made by each treating intensivist. Those who did not complete a session of IHD were excluded from the analysis of physiologic and biochemical parameters but included in the analysis of clinical outcomes.
Intermittent Hemodialysis
IHD was performed via a double-lumen hemodialysis catheter (12F, Gentle Cath; Medtronic, Dublin, Ireland) percutaneously inserted into either the internal jugular or femoral vein. A DBB-100NX dialysis machine (NIKKISO, Tokyo, Japan) and either a KF12 or KF15 synthetic hydrophilic membrane (Asahi Kasei Medical, Tokyo, Japan) was used. Blood and dialysate flow were 200 mL/min and 500 mL/min, respectively. The duration of one IHD session was 4 hours but extended up to 6 hours if deemed necessary. Unfractionated heparin or nafamostat mesilate (Torii Pharmaceutical, Tokyo, Japan) was administered for anticoagulation during IHD. For those with bleeding or high risk of bleeding, IHD was performed without anticoagulation.
Study Variables
Physiologic variables included systolic blood pressure (SBP), mean arterial pressure (MAP), heart rate (HR), and measured minute ventilatory volume (MV). Biochemical variables included pH, biocarbonate (Hco3–), Paco2, base excess, and lactate and were obtained from arterial blood gas analysis. These data were collected from the electronic medical records. Clinical outcome data, such as ICU mortality, in-hospital mortality, 28-day and 90-day mortality, ICU-free days and alive, RRT-free days and alive, ventilator-free days and alive at the 28th day were also retrieved from the electronic medical records.
Statistical Analysis
Data were analyzed descriptively. Continuous or categorical variables were described as medians and interquartile ranges (25–75th) or as counts and percentages, respectively.
It was not assumed that the continuous study variables were normally distributed; bootstrapping methods were used to estimate the means and CIs for differences in these variables before and after IHD. All statistical analyses were performed using R 3.4.1 (R Core Team 2017; R Foundation for Statistical Computing, Vienna, Austria.).
RESULTS
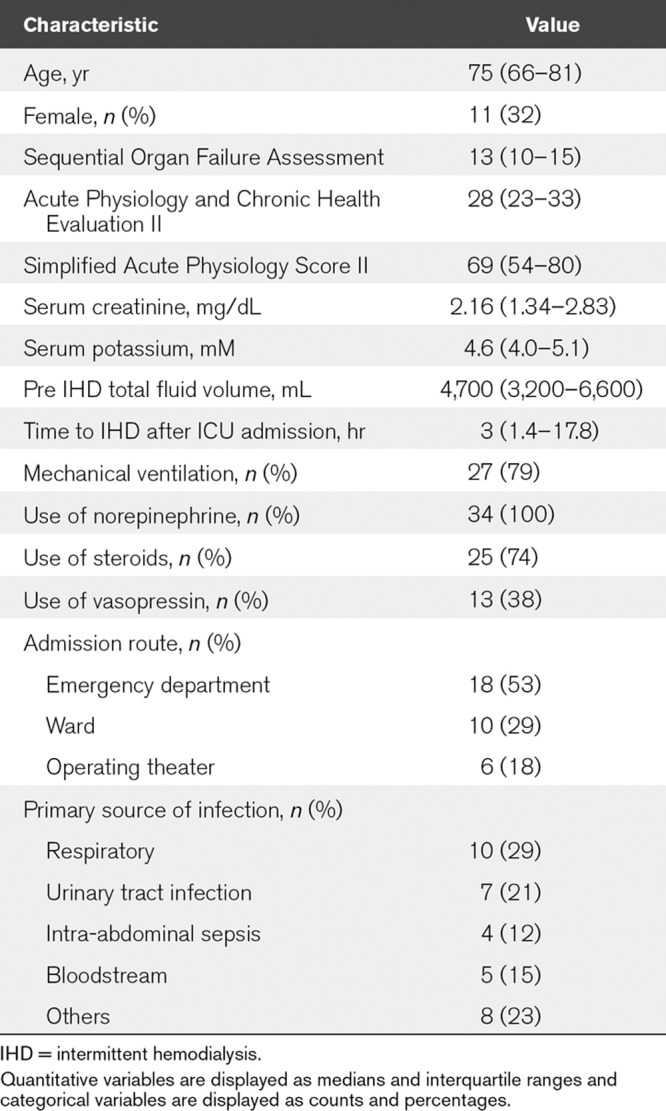
A total of 1,190 patients admitted to the ICU were screened for study eligibility. A total of 34 patients fulfilled the study inclusion criteria (Fig. 1). The baseline characteristics of the study population are shown in Table 1. Two patients (5.9%) had 1,000 mL of fluid removal. Only one patient (2.9%), who was an 81-year-old female with septic shock of unknown origin, failed to complete a planned session of IHD due to a decision to withdraw, which was made during IHD, followed by her immediate death.
Figure 1.
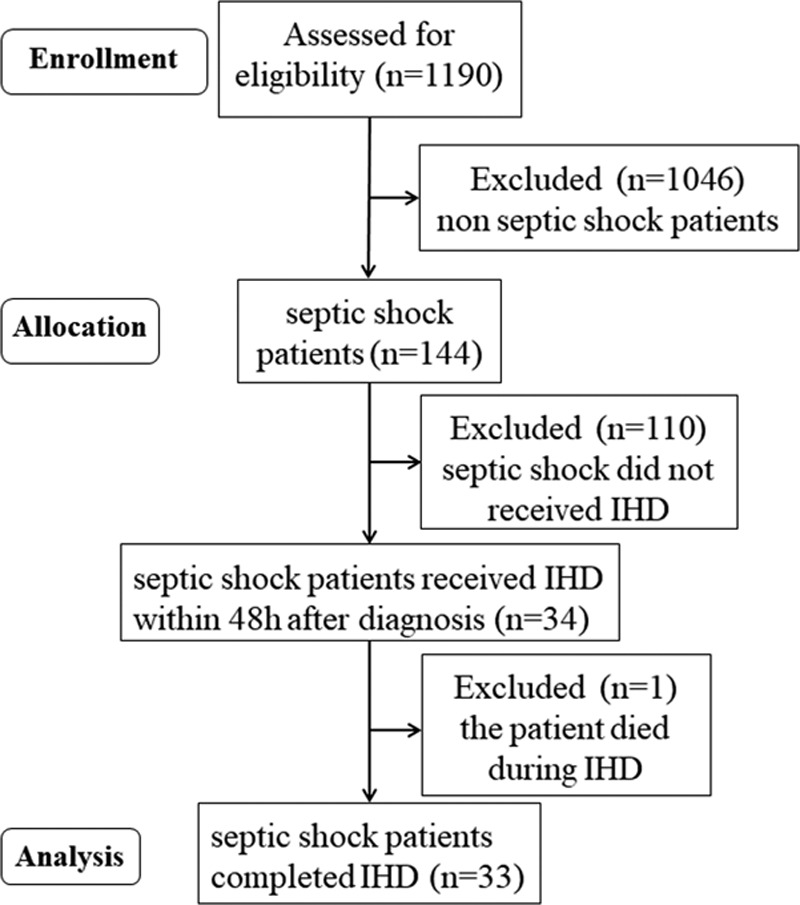
Flow chart of the study cohort. IHD = intermittent hemodialysis.
TABLE 1.
Baseline Characteristics of the Study Patients (n = 34)
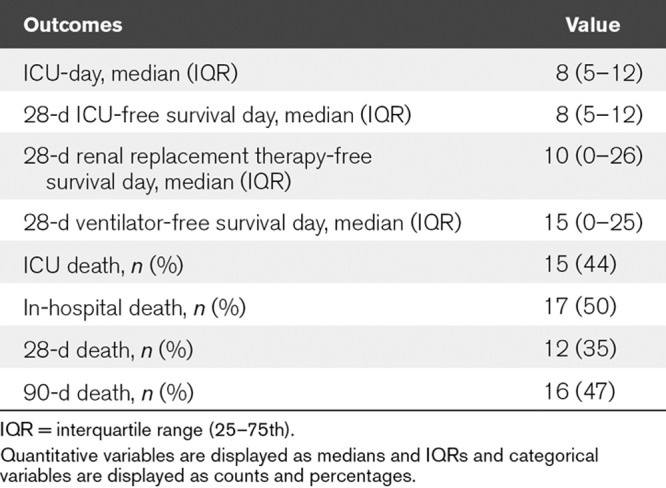
Regarding the physiologic parameters, an increase in MAP and a decrease in MV were observed after IHD. In contrast, there was no significant difference in HR before and after IHD. For biochemical parameters, increases in Hco3– and pH were observed after IHD (Table 2 and Fig. 2A–D). In addition, the noradrenaline requirement and inotropic score (11) decreased after IHD (Table 2). All patients completed the 90-day follow-up after the initiation of IHD. ICU mortality, in-hospital mortality, 28-day and 90-day mortality were 15 (44%), 17 (50%), 12 (35%), and 16 (47%), respectively. ICU-free days and alive, RRT-free days and alive, and ventilator-free days and alive on the 28th day were 8 (5–12), 10 (0–26), 15 (0–25), respectively (Table 3).
TABLE 2.
Comparison of Variables Before and After Intermittent Hemodialysis (n = 33)
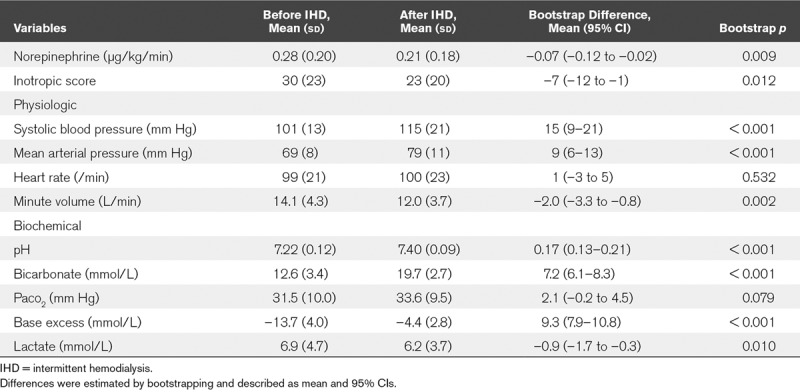
Figure 2.
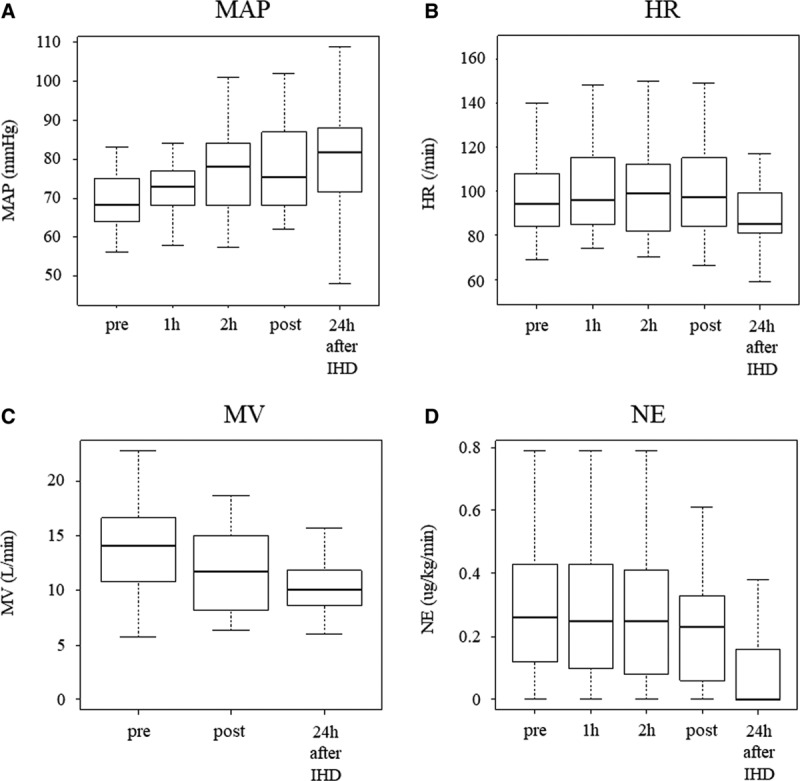
Changes in mean arterial pressure (MAP), heart rate (HR), measured minute ventilatory volume (MV), and norepinephrine (NE) over time after the start of intermittent hemodialysis (IHD).
TABLE 3.
Clinical Outcomes of Study Patients (n = 34)
DISCUSSION
In this study, it was observed that IHD in patients who had metabolic acidosis during resuscitation of septic shock was associated with improvement not only in biochemical parameters but also in respiratory and circulatory physiology with decreased norepinephrine requirement as compared with pre-IHD status.
Our findings raise a question regarding the consensus that IHD should be avoided for hemodynamically unstable patients including those who suffer from septic shock complicated by clinically significant metabolic acidosis. When providing RRT in hemodynamically unstable patients, the current common understanding is in favor of CRRT rather than IHD for fear of further deterioration of hemodynamics (12–17). A randomized controlled trial comparing IHD and CRRT in patients with septic shock complicated by acute kidney injury (AKI) showed an increase in SBP 2 hours after the initiation of CRRT, whereas a decrease in SBP at 0.5 hours and 2 hours after the initiation of IHD (5). However, the study population and the aim of RRT in the study were different from those of our study, in which IHD was performed in order to manage metabolic acidosis in patients with septic shock while being resuscitated.
Serum lactate level after IHD in the study population decreased slightly, although it remained at a high level. In a previous study, lactate clearance was inversely associated with increased risk of death over the first 6 hours after ICU admission (18). However, data on lactate clearance by RRT are limited and conflicting (19). Some researchers suggest that increased lactate clearance may be the improved endogenous acid-base and metabolic status achieved during RRT rather than the removal of large amounts of lactate by RRT (20). We assumed that improvement of circulation was associated with a slightly decreased lactate level and IHD may contributed to hemodynamic stabilization. Significant acidosis is associated with catecholamine-refractory hypotension through a mechanism that involves a decreased number of β adrenergic receptors and decreased myocardial contractility (21, 22). In contrast, improvement of metabolic acidosis is associated with an enhanced effect of catecholamines and an alleviated effort for respiratory compensation.
To artificially compensate metabolic acidosis in hemodynamically unstable septic shock patients, CRRT is generally preferred to IHD. Current AKI guidelines recommend delivering CRRT at an effluent volume of 20–25 mL/kg/hr in patients with AKI (4). In Japan, however, CRRT is generally delivered at a lower intensity (12–16 mL/kg/hr, up to 800 mL/hr) than the recommended intensity because of limited daily maximum use of CRRT solutions covered by public health insurance (6). In addition, our ICU had limited access to CRRT during the study period. Although these rare environments forced us to use IHD to manage clinically significant metabolic acidosis during septic shock resuscitation, in our observation, IHD is a feasible and even beneficial therapeutic option to manage this condition. As the typical dose of IHD (500 mL/min = 30,000 mL/hr) is much higher than that of CRRT, comparison to CRRT is of particular interest. Other potential benefits of IHD as compared with CRRT include earlier mobilization of patients, less staff-time requirements to provide RRT, simpler antibiotic dosing, shorter and less exposure to anticoagulants, and lower costs associated with RRT.
Little is known about the safety and efficacy of IHD use in hemodynamically unstable patients with septic shock. This study described changes in physiologic and biochemical parameters and decreased norepinephrine requirement before and after IHD, and major clinical outcomes such as mortality and event-free days, in patients who received IHD for the management of severe metabolic acidosis while resuscitation of septic shock.
Our study has several limitations. First, our study was a single-center retrospective study that described within group, before IHD and after IHD changes in regard to physiologic and biochemical parameters without a control group. Second, decisions to initiate IHD for metabolic acidosis correction were made by attending intensivists without any formal objective criteria. Third, our study population was limited to very old Japanese patients (median age, 75 yr) who lived in a rural area. This may limit the generalizability of the study interpretation. Fourth, one patient, who withdrew during IHD followed by immediate death, was excluded for the analysis of physiologic and biochemical variables. This potentially caused selective bias toward favorable effects of our practice. Fifth, IHD efficiently removes most of the commonly used antibiotics such as β-lactams, potentially resulting in therapeutic underdose, although special attention to this issue was always paid in our ICU.
CONCLUSIONS
Favorable changes in physiologic and biochemical variables and norepinephrine dependency were observed after IHD in patients with septic shock complicated by metabolic acidosis during resuscitation. Further studies are needed to show feasibility and benefit of IHD for this purpose.
ACKNOWLEDGMENTS
We thank Kosuke Sekine for his support with the data collection process and useful discussions.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 201743304–377 [DOI] [PubMed] [Google Scholar]
- 2.Jentzer JC, Vallabhajosyula S, Khanna AK, et al. Management of refractory vasodilatory shock. Chest 2018154416–426 [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005294813–818 [DOI] [PubMed] [Google Scholar]
- 4.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012120c179–c184 [DOI] [PubMed] [Google Scholar]
- 5.John S, Griesbach D, Baumgärtel M, et al. Effects of continuous haemofiltration vs intermittent haemodialysis on systemic haemodynamics and splanchnic regional perfusion in septic shock patients: A prospective, randomized clinical trial. Nephrol Dial Transplant 200116320–327 [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Toki N, Takeda K, et al. ; Japanese Society for Physicians and Trainees in Intensive Care (JSEPTIC) Clinical Trial Group Validity of low-intensity continuous renal replacement therapy*. Crit Care Med 2013412584–2591 [DOI] [PubMed] [Google Scholar]
- 7.Kellum JA, Angus DC, Johnson JP, et al. Continuous versus intermittent renal replacement therapy: A meta-analysis. Intensive Care Med 20022829–37 [DOI] [PubMed] [Google Scholar]
- 8.Rabindranath K, Adams J, Macleod AM, et al. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev. 2007;3:CD003773. doi: 10.1002/14651858.CD003773.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw SM, Berthiaume LR, Delaney A, et al. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: A meta-analysis. Crit Care Med 200836610–617 [DOI] [PubMed] [Google Scholar]
- 10.Levy MM, Fink MP, Marshall JC, et al. ; International Sepsis Definitions Conference 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med 200329530–538 [DOI] [PubMed] [Google Scholar]
- 11.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 20093012445–2452 [DOI] [PubMed] [Google Scholar]
- 12.Pannu N, Klarenbach S, Wiebe N, et al. ; Alberta Kidney Disease Network Renal replacement therapy in patients with acute renal failure: A systematic review. JAMA 2008299793–805 [DOI] [PubMed] [Google Scholar]
- 13.Friedrich JO, Wald R, Bagshaw SM, et al. Hemofiltration compared to hemodialysis for acute kidney injury: Systematic review and meta-analysis. Crit Care. 2012;16:R146. doi: 10.1186/cc11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: A systematic review and meta-analysis. Intensive Care Med 201339987–997 [DOI] [PubMed] [Google Scholar]
- 15.Augustine JJ, Sandy D, Seifert TH, et al. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 2004441000–1007 [DOI] [PubMed] [Google Scholar]
- 16.Schefold JC, von Haehling S, Pschowski R, et al. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): A prospective randomized controlled trial. Crit Care. 2014;18:R11. doi: 10.1186/cc13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truche AS, Darmon M, Bailly S, et al. ; OUTCOMEREA Study Group Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: Impact on mortality and renal recovery. Intensive Care Med 2016421408–1417 [DOI] [PubMed] [Google Scholar]
- 18.Nichol A, Bailey M, Egi M, et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care. 2011;15:R242. doi: 10.1186/cc10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Corte W, Vuylsteke S, De Waele JJ, et al. Severe lactic acidosis in critically ill patients with acute kidney injury treated with renal replacement therapy. J Crit Care 201429650–655 [DOI] [PubMed] [Google Scholar]
- 20.Briglia AE. The current state of nonuremic applications for extracorporeal blood purification. Semin Dial 200518380–390 [DOI] [PubMed] [Google Scholar]
- 21.Marsh JD, Margolis TI, Kim D. Mechanism of diminished contractile response to catecholamines during acidosis. Am J Physiol 1988254H20–H27 [DOI] [PubMed] [Google Scholar]
- 22.Orchard CH, Hamilton DL, Astles P, et al. The effect of acidosis on the relationship between Ca2+ and force in isolated ferret cardiac muscle. J Physiol 1991436559–578 [DOI] [PMC free article] [PubMed] [Google Scholar]


