Abstract
Background
Alterations in mineral metabolism, such as high phosphorus, high parathyroid hormone (PTH), and high fibroblast growth factor-23 (FGF-23) have been identified as potential risk factors for heart failure (HF). Important differences in the prevalence of mineral metabolism abnormalities and in the risk of HF have been reported across race and/or ethnic groups. In this study, we evaluated whether the associations of mineral metabolism markers with HF differed by race and/or ethnicity.
Methods
We included participants free of cardiovascular disease from the Multi-Ethnic Study of Atherosclerosis to quantify rates of HF overall and across race and/or ethnic groups. Using Cox models, we tested associations of baseline higher phosphorus (>4 mg/dL), PTH greater than 65 pg/mL, and FGF-23 greater than 46.5 pg/mL with incident HF, and for interactions by race and/or ethnicity, adjusting for sociodemographic and cardiovascular risk factors.
Results
Among the 6413 participants, median follow-up time was 14.9 years. The incidence rate for HF was highest for African Americans and lowest for Chinese (4.71 and 2.42 per 1000 person-years, respectively). The prevalence of elevated PTH (18.8% vs 7.4%) but not FGF-23 (23.1% vs 28.8%) was higher in African Americans vs Whites. In multivariable models, the associations of elevated PTH (hazard ratio [HR] 1.50, 95% CI: 1.13-1.99) and FGF-23 (HR 1.37, 95% CI: 1.07-1.75) with incident HF were statistically significant. However, the interactions by race and/or ethnicity were not statistically significant.
Conclusions
In a multiethnic population, higher PTH and FGF-23 were associated with risk of HF in African American and Hispanic individuals. There is no evidence that race and/or ethnicity modifies the association of altered mineral metabolism with risk of HF.
Keywords: heart failure, fibroblast growth factor-23, parathyroid hormone, phosphorus, mineral metabolism
Heart failure (HF) is common and is associated with high morbidity and mortality (1). Alterations in mineral metabolism, such as high phosphorus, high parathyroid hormone (PTH), and high fibroblast growth factor-23 (FGF-23) have been identified as potential risk factors for HF (2-7).
Important differences in the prevalence of mineral metabolism abnormalities as well as in the risk of HF have been reported across race and/or ethnic groups (8, 9). For example, African Americans have a greater risk of HF than other race and/or ethnic groups and a high prevalence of certain mineral metabolism abnormalities (8, 10-12). It is unknown whether the association of mineral metabolism abnormalities and risk of HF differs across race and/or ethnic groups. Such a finding might give insight to potentially identify modifiable targets in subpopulations.
In this study, we examined the rates of HF across race and/or ethnic groups and evaluated whether the associations of mineral metabolism markers with HF differed across these groups. We hypothesized that the contribution of abnormal phosphorus, PTH, and FGF-23 with risk of HF would be stronger in African Americans than in other race and/or ethnic groups.
Methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a community-based prospective cohort study of clinical and subclinical cardiovascular disease (13). Between the years 2000 and 2002, MESA enrolled 6814 adults ages 45 to 84 years from 6 field centers (New York and Bronx counties, New York; Baltimore City and County, Maryland; Forsyth County, North Carolina; Chicago, Illinois; St. Paul, Minnesota; and Los Angeles, California). Only individuals without known prevalent clinical cardiovascular disease were eligible to participate. Each site’s institutional review board approved the study and all participants provided informed consent. For this study, we excluded 391 individuals who were missing mineral metabolism measures and 1 person with no follow-up data, leaving a sample size of 6413.
Mineral metabolism markers
We studied a panel of mineral metabolism markers including PTH, FGF-23, and phosphorus. Serum samples were collected in the morning at the baseline MESA exam in 2000 to 2002, after an overnight fast, and stored at –80°C. Intact serum PTH was quantified using a 2-site immunoassay on a Beckman Access2 clinical analyzer (Beckman-Coulter, Inc) (14). The reference range, determined from the central 95% of values from 43 laboratory personnel with normal 25-hydroxyvitamin D concentrations, is 17 to 65 pg/mL. The interassay coefficient of variation was 6.1% at 30.1 pg/mL and 3.4% at 94.5 pg/mL. Serum intact FGF-23 was measured using the Kainos sandwich immunoassay, which measures the full-length (intact) FGF-23 molecule by recognizing both midmolecule and distal epitopes (15). Interassay coefficients of variation for singlicate high and low control samples were 6.7% and 12.4%, respectively. Serum phosphorus was measured using a timed-rate colorimetry reaction. Categories of each mineral metabolism marker were determined by accepted clinical values or greater than and less than the median if clinical norms were not available (phosphorus >4 vs ≤4 mg/dL; FGF-23 >46.5 vs ≤46.5 pg/mL; and PTH >65 vs ≤65 pg/mL).
Outcomes
In addition to the follow-up MESA examinations, a telephone interviewer contacted each participant every 9 to 12 months to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. To verify self-reported diagnoses, copies were requested of death certificates and medical records for all hospitalizations and outpatient cardiovascular diagnoses. Next-of-kin interviews for out-of-hospital cardiovascular deaths were conducted. Cardiovascular disease outcomes were adjudicated by the MESA events committee, which included cardiologists, physician epidemiologists, and general clinicians (13, 16) who review the reports gathered by a team of trained individuals who interview participants by phone and gather the appropriate records on each of the reported events. Details regarding the MESA processes and criteria for identifying, verifying, classifying, and adjudicating cardiovascular events have been previously reported (11, 16, 17). MESA was successful in obtaining medical records for an estimated 98% of hospitalized cardiovascular events and information on 95% of outpatient cardiovascular diagnostic encounters. Follow-up telephone interviews were completed for 92% of living participants.
The end point evaluated in this study was a composite of probable and definite HF. Probable HF required clinical HF symptoms or signs, a physician diagnosis of HF, and medical treatment for HF. Definite HF additionally required pulmonary edema and/or congestion by chest radiograph, evidence of a dilated left ventricle or reduced left ventricular ejection fraction (EF) by echocardiography or ventriculography, or evidence of left ventricular diastolic dysfunction by echocardiography.
In secondary analyses, we characterized the HF event as either preserved EF HF (left ventricular ejection fraction [LVEF] ≥50%) or reduced EF HF [LVEF ≤50%], extracted from echocardiograms concomitant with the HF event.
Covariates
Covariates included age, race and/or ethnicity, sex, income, education, and smoking status, which were determined by self-report. Level of education was categorized as high school or less, some college /technical school certificate, and bachelor/associate/graduate or professional school degree. Income categories were defined as less than $20 000, $20 000 to $49 999, or greater than or equal to $50 000 gross family income. Body mass index (BMI) was calculated based on height and weight measured at the baseline study visit. Blood pressure was ascertained as the mean of the last 2 of 3 seated measurements. Diabetes was defined as a fasting blood glucose concentration of 126 mg/dL or greater or use of insulin or oral hypoglycemic medications. Glomerular filtration rate was estimated from the Chronic Kidney Disease (CKD)–Epidemiology Collaboration equation (eGFRCKD-EPI) (18). CKD was defined as eGFRCKD-EPI less than 60 ml/min/1.73 m2. Urinary albumin concentration was determined by nephelometry, using the Array 360 CE Protein Analyzer (Beckman Instruments, Inc). Data regarding vitamin D supplement use were not available.
Statistical methods
Participants were considered at risk from the MESA baseline exam date to the first occurrence of HF, or until their data are censored because of death, loss to follow-up, or end of follow-up. Rates of HF were calculated per 1000 person-years overall and across race and/or ethnic groups and categories of each mineral metabolism marker.
Cox proportional hazards regression models were used to estimate the relative hazard of HF across categories of each mineral metabolism marker overall and then stratified by race and/or ethnicity. Sensitivity analyses were conducted modeling mineral metabolism markers continuously, per doubling for the right-skewed FGF-23 and PTH (log-base 2 transformation) and per mg/dL for phosphorus. Race and/or ethnicity–specific HRs were calculated via linear combination of regression coefficients for main effect and cross-product terms. Multiplicative interactions with race and/or ethnicity were tested by the Wald test of a product term for each mineral metabolism marker and race and/or ethnicity categories. Model 1 included age, sex, race, income, and education. Model 2 additionally included BMI, systolic blood pressure, blood pressure medications, lipids, diabetes, smoking, and eGFR.
In sensitivity analyses, we calculated incidence rates and repeated our Cox models, stratifying by preserved EF or reduced EF HF. Multiple imputation using chained equations was used among participants with missing EF values (N = 85) (19). The multiple analyses over the imputations were combined using Rubin rules to account for the variability in the imputation procedure (20).
The population attributable risk percentage (PAR%) was calculated by combining the relative risks and the observed prevalence rates of the risk factors of interest (21). Briefly, the PAR% quantifies the theoretic proportion of HF cases that would be eliminated by removing the risk factor, if the risk factor were causally associated with HF risk.
Analyses were conducted using Stata 16.0 and R, version 3.4.1.
Results
Study population
Whites had higher educational levels and income compared with other racial/ethnic groups. BMI and systolic blood pressure were highest in African Americans and Hispanics, who were also more likely to have diabetes and be smokers (Table 1).
Table 1.
Baseline participant characteristics by race and/or ethnicity
| Race and/or ethnicity | ||||
|---|---|---|---|---|
| White | Chinese | African American | Hispanic | |
| No. of participants | 2492 | 783 | 1739 | 1399 |
| Demographic data | ||||
| Age, y | 62.6 (±10.3) | 62.4 (±10.4) | 62.1 (±10.1) | 61.3 (±10.4) |
| Female sex | 1305 (52.4) | 406 (51.9) | 971 (55.8) | 732 (52.3) |
| Study site | ||||
| Forsyth County, NC | 517 (20.7) | 0 (0.0) | 446 (25.6) | 3 (0.2) |
| New York and Bronx Counties, NY | 209 (8.4) | 2 (0.3) | 345 (19.8) | 452 (32.3) |
| Baltimore City and County, MD | 513 (20.6) | 0 (0.0) | 511 (29.4) | 0 (0.0) |
| St. Paul, MN | 578 (23.2) | 0 (0.0) | 0 (0.0) | 441 (31.5) |
| Chicago, IL | 549 (22.0) | 296 (37.8) | 288 (16.6) | 0 (0.0) |
| Los Angeles, CA | 126 (5.1) | 485 (61.9) | 149 (8.6) | 503 (36.0) |
| Highest level of education completed | ||||
| High school | 534 (21.5) | 318 (40.7) | 529 (30.6) | 915 (65.4) |
| Some college/technical school | 591 (23.8) | 110 (14.1) | 500 (28.9) | 298 (21.3) |
| College graduate | 1360 (54.7) | 354 (45.3) | 701 (40.5) | 186 (13.3) |
| Total gross family income | ||||
| < $20 000 | 263 (10.8) | 324 (41.6) | 355 (22.2) | 535 (39.0) |
| $20 000-$49 999 | 771 (31.8) | 231 (29.7) | 658 (41.1) | 587 (42.8) |
| ≥ $50 000 | 1391 (57.4) | 223 (28.7) | 587 (36.7) | 249 (18.2) |
| Physical examination | ||||
| Body mass index, kg/m2 | 27.7 (±5.0) | 24.0 (±3.3) | 30.1 (±5.8) | 29.5 (±5.1) |
| Systolic blood pressure, mmHg | 123.3 (±20.5) | 124.6 (±21.7) | 131.9 (±21.6) | 126.7 (±21.9) |
| Medical history | ||||
| Diabetes | 143 (5.7) | 100 (12.8) | 306 (17.6) | 243 (17.4) |
| Chronic kidney disease | 385 (15.5) | 96 (12.3) | 200 (11.5) | 133 (9.5) |
| Antihypertensive medication use | 651 (26.1) | 216 (27.6) | 836 (48.1) | 407 (29.1) |
| Lipid-lowering medication use | 458 (18.4) | 111 (14.2) | 292 (16.9) | 186 (13.3) |
| Smoking status | ||||
| Never | 1109 (44.6) | 590 (75.4) | 788 (45.5) | 749 (53.5) |
| Former | 1092 (43.9) | 148 (18.9) | 638 (36.9) | 454 (32.5) |
| Current | 285 (11.5) | 44 (5.6) | 304 (17.6) | 196 (14.0) |
| Labs | ||||
| Low-density lipoprotein, mg/dL | 117.0 (±30.1) | 115.0 (±29.1) | 116.2 (±33.1) | 119.3 (±32.4) |
| Total cholesterol, mg/dL | 195.8 (±35.1) | 192.6 (±31.9) | 189.4 (±36.5) | 197.9 (±37.1) |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 80.8 (±16.2) | 87.7 (±18.7) | 86.4 (±18.5) | 86.0 (±17.5) |
Continuous variables are presented as mean (±SD) and categorical variables as n (%).
Abbreviations: CA, California; IL, Illinois; MD, Maryland; MN, Minnesota; NC, North Carolina; NY, New York.
Overall, PTH was highest among African Americans, whereas FGF-23 was higher among Whites. There were no differences in phosphorus across race and/or ethnic groups (All supplementary material and figures are located in a digital research materials repository 22).
Risk of incident heart failure
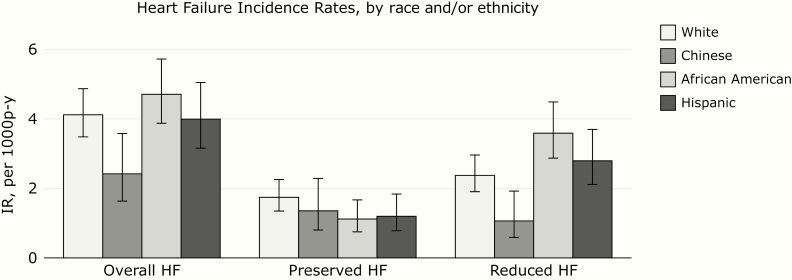
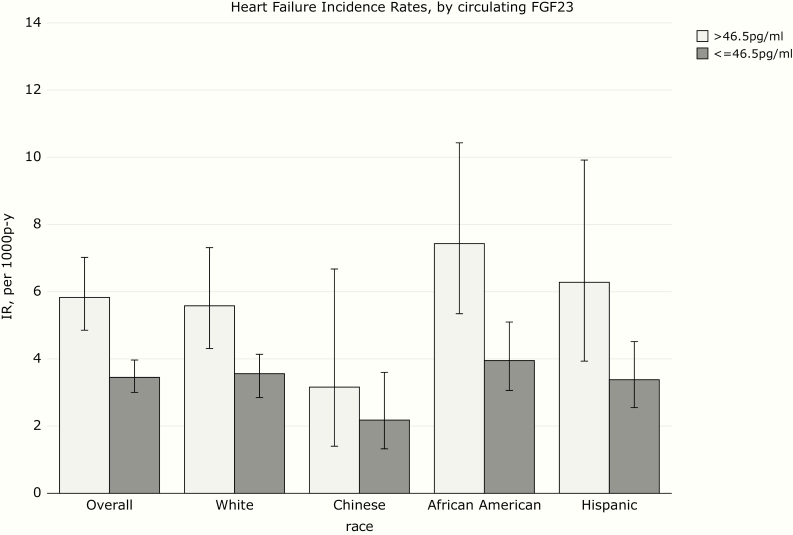
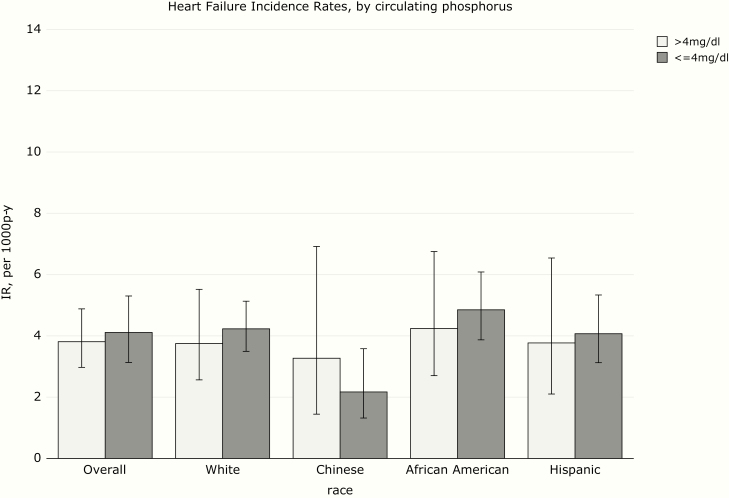
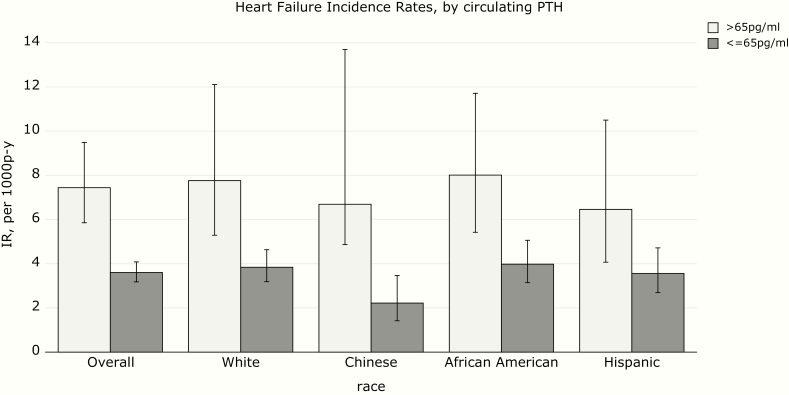
Over a median follow-up time of 14.9 years, there were 333 incident HF events (247 definite and 86 probable). The incidence rate for HF was highest for African Americans and lowest for Chinese (Figure 1). HF incidence rate was numerically higher in participants with higher FGF-23, particularly in African Americans and Hispanics (Figure 2). HF rates were similar among participants with high and low phosphorus (Figure 3), and much higher in participants with high PTH (Figure 4), with the highest rate seen in African Americans (Figure 1).
Figure 1.
Heart failure incidence rates (IRs) by race and/or ethnicity.
Figure 2.
Heart failure incidence rates (IRs) by circulating fibroblast growth factor-23 (FGF-23).
Figure 3.
Heart failure incidence rates (IRs) by circulating phosphorus.
Figure 4.
Heart failure incidence rates (IRs) by circulating parathyroid hormone (PTH).
Association of mineral metabolism measures with incident heart failure across race and/or ethnic groups
Associations of elevated PTH (HR 1.50, 95% CI: 1.13-1.99) and FGF-23 (HR 1.37, 95% CI: 1.07-1.75) but not phosphorus (HR 1.23, 95% CI: 0.92-1.63) with incident HF were statistically significant in multivariable models. However, the interactions by race and/or ethnicity were not statistically significant (Table 2). Similar findings were noted in analyses examining mineral metabolism markers continuously (22).
Table 2.
Association of mineral metabolism measures and incident heart failure, overall and by race and/or ethnicity
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Variable | HRa (95% CIb) | P | HR (95% CI) | P | HR (95% CI) | P |
| FGF-23 >46.5 vs ≤46.5 (ref) | ||||||
| Overall | 1.71 (1.36-2.14) | <.01 | 1.58 (1.25-2.00) | <.01 | 1.37 (1.07-1.75) | .01 |
| White | 1.58 (1.12-2.22) | .01 | 1.39 (0.98-1.97) | .06 | 1.20 (0.84-1.71) | .31 |
| Chinese | 1.45 (0.63-3.36) | .38 | 1.35 (0.59-3.13) | .48 | 1.23 (0.53-2.87) | .63 |
| African American | 1.89 (1.25-2.85) | <.01 | 1.85 (1.19-2.88) | <.01 | 1.55 (0.98-2.47) | .06 |
| Hispanic | 1.86 (1.11-3.12) | .02 | 1.80 (1.07-3.04) | .03 | 1.63 (0.96-2.76) | .07 |
| P for interaction | .87 | .71 | .72 | |||
| Phosphorus >4 vs ≤4 (ref) | ||||||
| Overall (race-adjusted) | 0.93 (0.72-1.22) | .61 | 1.25 (0.94-1.66) | .12 | 1.23 (0.92-1.63) | .17 |
| White | 0.89 (0.58-1.34) | .57 | 1.13 (0.74-1.74) | .57 | 1.13 (0.74-1.75) | .57 |
| Chinese | 1.51 (0.65-3.49) | .34 | 2.30 (0.99-5.32) | .05 | 2.31 (1.00-5.35) | .05 |
| African American | 0.87 (0.53-1.44) | .60 | 1.29 (0.77-2.17) | .33 | 1.25 (0.74-2.11) | .41 |
| Hispanic | 0.92 (0.52-1.64) | .79 | 1.15 (0.64-2.05) | .65 | 1.08 (0.60-1.94) | .79 |
| P for interaction | .73 | .54 | .51 | |||
| PTH >65 vs ≤65 (ref) | ||||||
| Overall (race-adjusted) | 2.01 (1.53-2.63) | <.01 | 1.84 (1.39-2.44) | <.01 | 1.50 (1.13-1.99) | <.01 |
| White | 2.03 (1.25-3.31) | <.01 | 1.81 (1.13-2.92) | .01 | 1.52 (0.96-2.41) | .07 |
| Chinese | 3.02 (0.91-10.01) | .07 | 2.88 (0.81-10.27) | .10 | 2.33 (0.65-8.29) | .19 |
| African American | 2.02 (1.32-3.09) | <.01 | 1.83 (1.15-2.92) | .01 | 1.39 (0.86-2.25) | .18 |
| Hispanic | 1.82 (1.05-3.15) | .03 | 1.75 (1.01-3.03) | .04 | 1.51 (0.87-2.60) | .14 |
| P for interaction | .91 | .92 | .91 | |||
Model 1 includes age, sex, gross family income in the past 12 months, educational attainment, and in analyses including all participants, race and/or ethnicity. Model 2 includes model 1 and adds body mass index, systolic blood pressure, use of antihypertensive medication, low-density lipoprotein, total cholesterol, diabetes status, smoking status, and estimated glomerular filtration rate.
Abbreviations: FGF-23, fibroblast growth factor-23; HR, hazard ratio; PTH, parathyroid hormone; ref, reference.
aHazard ratio from Cox proportional hazards regression model evaluating the hazard of heart failure among individuals with elevated circulating mineral metabolism levels to those without.
bConfidence interval.
Association of kidney measures with incident heart failure subtypes race and/or ethnic groups
There were 117 HF events with preserved EF. Rate of preserved EF HF was higher in magnitude among Whites than in other race and/or ethnic groups (Figure 1). High FGF-23 in Whites and African Americans were associated with the hazard of preserved EF HF (22). No differences in associations of mineral metabolism markers with preserved EF HF were observed between racial/ethnic groups.
There were 216 HF events with reduced EF. High PTH in African Americans was significantly associated with greater hazard of reduced EF HF. No differences in associations of mineral metabolism markers with reduced EF HF were observed between racial/ethnic groups (22).
Population attributable risk of mineral metabolism markers for heart failure in African Americans and Whites
The prevalence of mineral metabolism abnormalities was higher in African Americans vs White participants. Consequently, the PAR% for high PTH, high FGF-23, and high phosphorus was higher for African Americans (12.07%, 16.25%, and 3.25%, respectively) than for Whites. These PAR%s were comparable to other important risk factors in African Americans, such as older age, high BMI, and diabetes (22).
Discussion
In this study of well-characterized, racially and/or ethnically diverse participants free of baseline cardiovascular disease, elevated PTH, phosphorus, and FGF-23 were significantly associated with incident HF. Furthermore, elevated FGF-23 was associated with preserved EF HF and elevated PTH with reduced EF HF. We found no differences in the association of mineral metabolism abnormalities with incident HF between race and/or ethnic groups. These data support the possible contribution of altered mineral metabolism to the development of HF, independent of race and/or ethnicity.
We found that the incidence rate for HF overall was highest in African Americans and lowest in Chinese compared with other race and/or ethnic groups. A previous analysis of MESA data (with shorter follow-up time) also reported high rates of incident HF among African Americans and Hispanic participants compared with other race and/or ethnic groups (11). Similarly, in the CARDIA study, the cumulative incidence of HF before age 50 years was substantially more common among African American men and women than in Whites (23). Studies have also reported higher rates of HF readmissions among African American and Hispanic participants (24, 25). In our study, African Americans in particular had higher rates of reduced EF HF, whereas rates of preserved EF HF were relatively similar across race and/or ethnic groups. This is consistent with a recent publication from the Atherosclerosis Risk in Communities study, which also reported higher rates of reduced HF EF in African American men and higher rates of preserved HF EF in white women (26). Our study adds to the body of literature that highlights important race and/or ethnic disparities in risk of HF.
African Americans and Hispanics had a greater prevalence of hyperparathyroidism and lower prevalence of elevated FGF-23. Other studies have reported similar findings for PTH and FGF-23 in African Americans. In a study of hemodialysis patients, African Americans were noted to have higher levels of PTH compared with other race and/or ethnic groups (27). In another study of hemodialysis patients, PTH levels were higher; however, FGF-23 was lower among African American than among non-African American patients (28). A study of advanced CKD patients also noted lower FGF-23 and higher PTH among African Americans vs Whites (29). In NHANES, African Americans and Mexican Americans were reported to have higher PTH compared with whites (12). Our studies extend and confirm the findings noted in these previous studies.
Contrary to our hypothesis, we did not find that the associations of altered mineral metabolism with HF differed across racial/ethnic subgroups. It is plausible that the findings may differ in other patient populations, for example, those with CKD. Given the rapidly growing population of HF patients in the United States, focusing on targeted interventions in high-risk subgroups would potentially have tremendous public health impact. Thus, further investigation is needed to identify modifiable HF risk factors in high-risk racial/ethnic subgroups.
Our study has several strengths. We studied a large, well-characterized, racially/ethnically diverse population with longitudinal follow-up. HF was ascertained by a rigorous physician-adjudication process. We recognize some limitations as well. Only baseline measures of mineral metabolism markers were available. There were few HF events among Chinese participants, so we may have been underpowered to detect significant associations in this racial group. Additionally, we were underpowered to examine HF events according to subtypes.
In conclusion, alterations in mineral metabolism markers, more specifically elevated PTH, are more prevalent in African American participants compared with Whites. However, there was no statistical evidence that the associations of altered mineral metabolism with risk of incident HF differed across racial/ethnic groups. These findings should be confirmed in other high-risk patient populations. Further studies are needed to investigate modifiable risk factors that may decrease the burden of HF among racial/ethnic minorities.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial Support: This work was supported by the National Heart, Lung, and Blood Institute (Contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01- HC-95164, N01-HC-95165, N01-HC-95166, N01- HC-95167, N01-HC-95168, and N01-HC-95169), the National Center for Advancing Translational Sciences (Grant UL1-TR-000040, Grant UL1-TR-001079, and Grant UL1-TR-001420), and the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01DK088762), (Grant R01DK099199), and Grant (K01DK109019).
Glossary
Abbreviations
- FGF-23
fibroblast growth factor-23
- HF
heart failure
- LVEF
left ventricular ejection fraction
- PTH
parathyroid hormone
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. [DOI] [PubMed] [Google Scholar]
- 2. Bansal N, Zelnick L, Robinson-Cohen C, et al. Serum parathyroid hormone and 25-hydroxyvitamin D concentrations and risk of incident heart failure: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3(6):e001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schierbeck LL, Jensen TS, Bang U, Jensen G, Køber L, Jensen JE. Parathyroid hormone and vitamin D—markers for cardiovascular and all cause mortality in heart failure. Eur J Heart Fail. 2011;13(6):626–632. [DOI] [PubMed] [Google Scholar]
- 4. Ix JH, Katz R, Kestenbaum BR, et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol. 2012;60(3):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kestenbaum B, Katz R, de Boer I, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14):1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu LC, Voors AA, van Veldhuisen DJ, et al. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail. 2011;13(6):619–625. [DOI] [PubMed] [Google Scholar]
- 7. Jovanovich A, Ix JH, Gottdiener J, et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231(1):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Boer IH, Gorodetskaya I, Young B, Hsu CY, Chertow GM. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol. 2002;13(11):2762–2769. [DOI] [PubMed] [Google Scholar]
- 9. Gupta A, Kallenbach LR, Zasuwa G, Divine GW. Race is a major determinant of secondary hyperparathyroidism in uremic patients. J Am Soc Nephrol. 2000;11(2):330–334. [DOI] [PubMed] [Google Scholar]
- 10. Bansal N, Katz R, Robinson-Cohen C, et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2016;2(3):314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 14. Bosworth C, Sachs MC, Duprez D, et al. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf). 2013;79(3):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kestenbaum B, Sachs MC, Hoofnagle AN, et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7(3):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52(25):2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan RT, Bluemke D, Gomes A, et al. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;57(17):1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 20. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. [DOI] [PubMed] [Google Scholar]
- 21. Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18(5):571–579. [DOI] [PubMed] [Google Scholar]
- 22. Robinson-Cohen C, Shlipak M, Sarnak M, et al. Impact of race on the association of mineral metabolism with heart failure: the Multi-Ethnic Study of Atherosclerosis, Supplemental Tables 2019. Deposited October 19, 2019. ProMED-mail website. https://github.com/cassyrc/mesa_race_hf.
- 23. Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360(12):1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail. 2016;4(11):885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vivo RP, Krim SR, Liang L, et al. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3(5):e001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014): ARIC Study Community Surveillance. Circulation. 2018;138(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalantar-Zadeh K, Miller JE, Kovesdy CP, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25(12):2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scialla JJ, Parekh RS, Eustace JA, et al. Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol. 2015;42(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jovanovich A, Chonchol M, Cheung AK, et al. ; HOST Investigators Racial differences in markers of mineral metabolism in advanced chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(4):640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]