Abstract
Background:
Breast reconstruction (BR) is the reconstructive surgical technique that focuses on restoring normal form and function to the breast following oncologic resection. The goal of this study was to determine if BR disparities exist among rural female patients in Kentucky.
Methods:
A retrospective (2006 – 2015), population-based cohort study was conducted on breast cancer patients (stages I-III) treated with mastectomy with or without BR. We used 2013 Beale codes to stratify patients according to geographic status. Chi-square tests were used to examine the association of BR along the rural-urban continuum. A multivariate logistic regression model controlling for patient, disease, and treatment factors was used to predict BR. The likelihood of BR was reported in odds ratios (OR) using a 95% confidence interval (CI).
Results:
Overall, 10,032 patients met study criteria. Of those, 2,159 (21.5%) underwent BR. The rate of BR among urban, near-metro, and rural patients was 31.1%, 20.4%, and 13.4%, respectively (P < .001). Multivariate analysis revealed that women from near metro (OR 0.54, CI: 0.47–0.61; P < .001) and rural areas (OR 0.36, CI: 0.31–0.41; P < .001) were less likely to undergo BR than women from urban areas.
Conclusion:
Although BR benefits are well documented, women from rural Kentucky undergo BR at lower rates and are less likely to receive BR than their urban counterparts. Efforts should seek to promote equitable access to BR for all patients, including those from rural areas.
Keywords: breast cancer, breast reconstruction, health care disparities, rural health
Breast cancer is the most common type of cancer in women regardless of age or ethnicity.1 The treatment for breast cancer is multidisciplinary in nature and may include the need for chemotherapy, radiation, hormonal therapy, immunotherapy or extirpative therapy, such as breast conserving surgery (eg, lumpectomy) or oncologic resection (eg, mastectomy). The negative sequalae of mastectomy includes significant psychosocial issues such as a negative self-image, anxiety, and depression.2–5 Post-mastectomy breast reconstruction (BR) is the reconstructive surgical technique that focuses on restoring normal form and function to the breast following oncologic resection (eg, mastectomy).6 Reconstructive approaches focus on the utilization of autologous tissue or may be implant-based.7,8 Regardless of the reconstructive technique, it is well established that BR is associated with significant improvements in body image, self-esteem, sexuality, quality of life, and psychosocial functioning.9,10
The Women’s Health Care and Cancer Rights Act of 1998 mandated that all insurers, including Medicare and Medicaid, cover the cost associated with BR.11 Coverage also includes surgery on the contralateral or non-diseased breast in order to achieve symmetry. More recently, the Breast Cancer Patient Education Act of 2015 was signed into law which mandates the Secretary of Health and Human Services to implement an education campaign to inform breast cancer patients about the availability and coverage of BR postmastectomy.12 Yet, less than half of women that require mastectomy are even offered BR, despite the documented benefits of BR and federally mandated coverage and education efforts.13 This suggests that more than health care coverage is a factor in receiving BR. As such, significant disparities in BR utilization have been reported, particularly with respect to age, race, insurer type, and distance to a plastic surgeon. Recent but limited evidence has also suggested that geographic area (eg, rural vs urban status) may also play an important role in receiving BR.14
According to the U.S. Health Resources and Services Administration, 88 of the 120 counties in Kentucky carry a health professional shortage area (HSPA) designation and are classified as non-metro.15 While HSPA does not take into account physician subspecialists including reconstructive surgeons, it highlights the fact that much of non-metro Kentucky lacks access to routine medical services. Furthermore, Appalachia Kentucky, a region that is challenged by socioeconomic and health care disparities, comprises 54 of Kentucky’s 120 counties.16 As such, existing studies on breast reconstruction disparities in rural populations may not be generalizable to rural Kentuckians. Therefore, the overall goal of this study was to determine if a disparity in BR utilization exists in women from rural Kentucky. We hypothesized that women from rural areas of the state undergo BR at lower rates and are less likely to undergo BR following oncologic resection for breast cancer relative to their urban and suburban counterparts.
Patients and Methods
To test our hypothesis, a retrospective population-based study was conducted from January 1, 2006, to December 31, 2015. This study was approved by the Institutional Review Board at the University of Kentucky.
Data Sources
The Kentucky Cancer Registry (KCR) was used to identify patients diagnosed with breast cancer and treated with mastectomy within the study timeframe. KCR is a prospectively maintained database and 1 of 18 cancer registries that report aggregated cancer data to the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database. The limitations of SEER with regards to reporting of BR have previously been described.17 KCR has received the highest level of certification (Gold) from the North American Association of Central Cancer Registries regarding the quality of data found within the registry. De-identified patient-level data were abstracted from KCR. Data included patient age at diagnosis, race (white, African American, all other), primary payer status (private, Medicaid, Medicare, uninsured, TRICARE/VA, unknown), American Joint Committee on Cancer (AJCC) pathologic stage (I-III, unknown), radiation status, BR status, and U.S. Department of Agriculture (USDA) rural-urban continuum codes (1–9).
Statistical Analysis
The primary outcome of this study was BR utilization rates across the rural-urban continuum in the state of Kentucky. Secondary outcomes included the likelihood of receiving BR and factors associated with the receipt of BR. Patients were divided into mastectomy-only and mastectomy plus BR groups. The 2013 USDA rural-urban continuum codes were used to stratify patients according to urban (1–2), near-metro (3–6) or rural (7–9) status. Chi-square tests were used to examine the association of BR along the rural-urban continuum. A quintile-ish breakdown of BR rates across the continuum was used to construct a heat map depicting BR rates by geographic status. A multivariate logistic regression model controlling for patient, disease, and treatment factors was used to predict BR. The likelihood of undergoing BR was reported in odds ratios (OR) using a 95% confidence interval (CI). Model goodness-of-fit was tested using the Hosmer-Lemeshow goodness-of-fit test. Patient characteristics were compared by geographic region (urban, near-metro, rural). Categorical variables were reported as frequencies (N) and column percentages (%) and were compared using chi-square tests. Continuous variables were tested for normality using the Shapiro-Wilk test for normality and histograms. Normally distributed continuous variables were reported using means and standard deviations (SD) and compared using t-tests and ANOVAs. Significance was set at a P value of P ≤ .05. All statistical analyses were performed using R programming language, version 3.4.3 (Austria, Vienna: R Core Team).
Results
Patient Demographics and Case Characteristics
Patient demographics and case characteristics are summarized in Table 1. Overall, 10,032 patients met study criteria and were included in the final analysis. Briefly, the mean age was highest in rural patients (62.1 years), followed by near-metro (61.3 years) and urban (60.8 years) (P < .001). Not surprisingly, differences (P < .001) in racial distribution across the rural-urban continuum were observed. White was the predominate race, followed by African American and all other races. As expected, private insurance was most common among urban patients, followed by near metro and rural (P < .001). Conversely, Medicare, Medicaid, and being uninsured was more common in women from near metro and rural areas. Finally, differences (P < .001) with respect to pathologic stage were also observed across the continuum.
Table 1.
Patient Demographics and Case Characteristics Stratified by Geographic Area.
| Overall | Geographic Area | P | |||
|---|---|---|---|---|---|
| Urban | Near-Metro | Rural | |||
| Total Number of Cases, n | 10,032 | 3,215 | 3,506 | 3,311 | |
| Age at Diagnosis | |||||
| Mean ± SD | 61.4 ± 13.6 | 60.8 ± 13.9 | 61.3 ± 13.4 | 62.1 ± 13.3 | < .001 |
| Race, n (%) | < .001 | ||||
| White | 9,332 (93.0) | 2,844 (88.5) | 3,266 (93.2) | 3,222 (97.3) | |
| African American | 617 (6.2) | 341 (10.6) | 204 (5.8) | 72 (2.2) | |
| Other | 83 (0.8) | 30 (0.9) | 36 (1.0) | 17 (0.5) | |
| Payer Status, n (%) | |||||
| Private | 4,544(45.3) | 1,681 (52.3) | 1,628 (46.4) | 1,235 (37.3) | < .001 |
| Medicaid | 4,316 (43.0) | 1,275 (39.7) | 1,478 (42.2) | 1,563 (47.2) | |
| Medicare | 829 (8.3) | 190 (5.9) | 260 (7.4) | 379 (11.4) | |
| Uninsured | 186 (1.9) | 35(1.1) | 67 (1.9) | 84 (2.5) | |
| TRICARE/VA | 99 (1.0) | 28 (0.9) | 45 (1.3) | 26 (0.8) | |
| Unknown | 58 (0.6) | 6 (0.2) | 28 (0.8) | 24 (0.7) | |
| AJCC Stage, n (%) | |||||
| Stage I | 3,952 (39.4) | 1,289 (40.1) | 1,446 (41.2) | 1,217 (36.8) | < .001 |
| Stage II | 4,029 (40.2) | 1,310 (40.7) | 1,379 (39.3) | 1,340 (40.5) | |
| Stage III | 1,838 (18.3) | 574 (17.9) | 603 (17.2) | 661 (20.0) | |
| Unknown | 213 (2.1) | 42 (1.3 | 78 (2.2) | 93 (2.8) | |
| Radiation Status, n (%) | 1,991 (19.8) | 721 (22.4) | 633 (18.1) | 637 (19.2) | < .001 |
| Breast Reconstruction, n (%) | 2,159 (21.5) | 1,001 (31.1) | 715 (20.4) | 443 (13.4) | < .001 |
Rural Women Undergo BR at Lower Rates Than Their Urban Counterparts
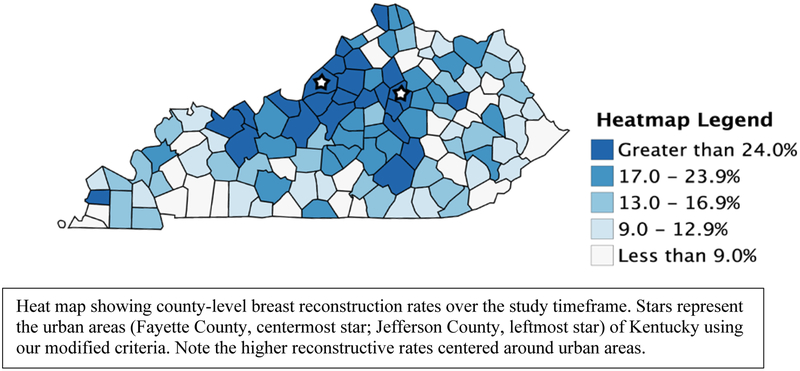
Overall, 2,159 (21.5%) patients underwent BR over the study timeframe. The individual rates of BR for urban, near-metro, and rural areas were 31.1%, 20.4%, and 13.4%, respectively (P < .001). A heatmap showing BR rates across the rural-urban continuum is shown in Figure 1.
Figure 1.
County-level Breast Reconstruction Rates in Kentucky (2006 – 2015)
Rural Women Are Less Likely to Receive BR
Results from the multivariate logistic regression model are summarized in Table 2. When holding all other variables constant, the regression model showed that rural-urban status was highly predictive of BR. Rural patients were 64.0% less likely (OR 0.36, CI: 0.31–0.41; P < .001) to undergo BR, while near-metro patients were 46.0% less likely (OR 0.54, CI: 0.47–0.61; P < .001) to undergo BR when compared to patients from urban areas. When comparing patients according to race, African American women (OR 0.76, CI: 0.60–0.95; P < .016) were less likely to undergo BR than their white counterparts. Payer type was also found to be predictive of BR. More specifically, patients with Medicaid (OR 0.11, CI: 0.09–0.13; P < .001), Medicare (OR 0.47, CI: 0.39–0.57; P < .001), or no insurance (OR 0.22, CI: 0.13–0.36; P < .001) were less likely to receive BR than patients with private insurance. Not surprisingly, advancing AJCC pathologic stage (stage II [OR 0.69, CI: 0.61–0.77], stage III [OR 0.33, CI: 0.27–0.40]) decreased the likelihood of receiving BR (P < .001). As expected, radiation status (OR 0.77, CI: 0.66–0.91; P < .002) was found to be a negative predictor of BR. Although increasing age is a negative predictor of BR, it was excluded from the model given the high correlation between age greater than the median and Medicare eligibility.
Table 2.
Multiple Logistic Regression Model for Breast Reconstruction Controlling for Race, Primary Payer Status, AJCC Stage, Radiation Status and Geography. N = 10,032.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Race | |||
| White | ref. | ref. | ref. |
| Black | 0.76 | 0.60 – 0.95 | .016 |
| Other | 1.57 | 0.96 – 2.54 | .068 |
| Primary Payer | |||
| Private | ref. | ref. | ref. |
| Medicaid | 0.11 | 0.10 – 0.126 | < .001 |
| Medicare | 0.47 | 0.39 – 0.57 | < .001 |
| Uninsured | 0.22 | 0.13 – 0.36 | < .001 |
| TRICARE/VA | 1.27 | 0.83 – 1.92 | .275 |
| Unknown | 0.25 | 0.09 – 0.54 | .001 |
| AJCC Stage | |||
| I | ref. | ref. | ref. |
| II | 0.69 | 0.61 – 0.77 | < .001 |
| III | 0.33 | 0.27 – 0.40 | < .001 |
| Unknown | 0.56 | 0.37 – 0.85 | < .007 |
| Radiation Used | 0.77 | 0.66 – 0.91 | .002 |
| Geography | |||
| Urban | ref. | ref. | ref. |
| Near-Metro | 0.54 | 0.47 – 0.61 | < .001 |
| Rural | 0.36 | 0.31 – 0.41 | < .001 |
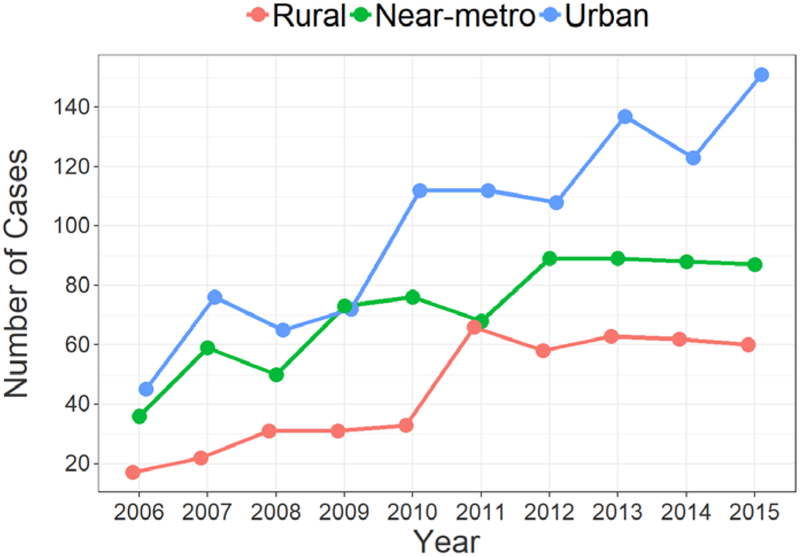
BR Rates Increase Over Time Across the Continuum
Trends in BR over the study period (2006 – 2015) were also examined and a positive correlation between year of diagnosis and BR utilization was found across the rural-urban continuum (Figure 2). However, the strength of the correlation differed significantly for urban (r = 0.229; P < .001), near-metro (r = 0.078; P < .001), and rural patients (r = 0.11; P < .001). BR in urban patients increased from 15.5% at the outset of the study to 50.7%, while near-metro increased from 20.4% to 24.9%, and rural increased from 6.3% to 17.8%.
Figure 2.
Breast Reconstruction Rates by Geographic Region (N = 10,032)
Discussion
Mastectomy is a life-saving treatment and an essential part of breast cancer care.18 However, it does not come without a cost. Post-mastectomy patients experience significant psychosocial issues including a negative self-image, anxiety, and depression—all of which have been shown to improve with BR.19 The WHRCA mandated that all health care plans, including Medicare and Medicaid, provide coverage for BR. Despite this mandate and the benefits of BR, disparities still exist in the utilization of BR, particularly among African American women, Hispanic women, and women from rural areas.14,20,21
To further examine our original hypothesis regarding BR utilization within Kentucky, we conducted a population-based cohort study using a prospectively maintained, high-quality cancer database. Consistent with the stated hypothesis, this study found significant differences in BR utilization among women from rural Kentucky. Women from rural areas underwent BR at lower rates and were less likely to undergo post-mastectomy BR than women from urban areas. These data are reinforced by the heatmap primarily showing clustering of county-level BR rates around metropolitan areas. Collectively, these data highlight a significant disparity in BR practices for women in rural Kentucky. It is important to note that significant differences in baseline characteristics were observed in our sample. To control for these differences and to examine the likelihood of undergoing BR across the continuum, we constructed a multivariate logistic regression model as described above. Consistent with prior studies,20,22–26 our model showed that patients who were of white race, lived in urban or near-metro areas, and were privately insured were more likely to undergo BR than those that were not. Other negative predictors of BR in our study included advancing pathologic stage and receipt of post-mastectomy radiation therapy.
Tseng and colleagues retrospectively reviewed 3,552 mastectomy cases from a SEER database in order to examine BR utilization in the greater Sacramento area.14 They found that rural area breast cancer patients were 27% less likely to undergo BR than their urban counterparts. Our study demonstrated that BR occurs at a much lower rate (13.4%) in women from rural Kentucky and that they were 64% less likely to undergo BR. While our findings coincide with that of Tseng and colleagues, our data show that rural Kentuckians undergo BR at much lower rates than what is reported in the literature for other rural areas. This suggests that additional factors specific to rural Kentucky patients may be contributory and this highlights the importance of focused, rural-urban research and that rural health care data from other parts of the country may not always be generalizable.
Interestingly, our study also found that BR utilization increased across the continuum over the study timeframe (2006 – 2015). Lang and associates investigated nationwide BR rates using SEER following enactment of WHCRA.27 They found that the rate of immediate BR increased over the study timeframe (1998 – 2008) from 11.7% to 21.7% However, their study did not take into account rural-urban differences. The observed changes in our study may be attributed in part to legislative efforts including Medicaid expansion under the Affordable Care Act. Mahmoudi and associates studied BR rates following Medicaid expansion in New York from 1998 – 2006 and found that the rate of BR was most pronounced for Medicaid enrollees.28 It is important to note, however, that Medicaid expansion in our study did not occur until the end of the study period (2014). While it may be responsible for some of the sustained increases in BR, it does not account for the initial inflections in BR rates that occurred around 2009 – 2010.
The Institute of Medicine has defined a safety net hospital as a hospital that “delivers a significant level of both health care and other health-related services to the uninsured, Medicaid, and other vulnerable populations.”29 Although a disparity in BR rates exists between safety net and private hospitals, Ballard and colleagues found that BR rates at safety net hospitals have continued to increase over time.30 The University of Kentucky is a safety net hospital located in Lexington, Kentucky, that provides medical care including comprehensive breast cancer care for a large part of the state. In 2013, the University of Kentucky’s Markey Cancer Center received a designation from the National Cancer Institute (NCI), becoming the only NCI-designated cancer center in the state. NCI designation carries with it access to experimental cancer treatments and among many other things, access to reconstructive breast services for a large portion of the state.31 This may also be responsible for some of the latter trends in the data. Nevertheless, despite the overall increase in BR, disparities in BR utilization remained for women in rural Kentucky. Collectively, these data suggest that while expanded health insurance coverage in conjunction with other factors may have improved access to care, there are other barriers that still remain.
Several barriers to BR have been well-documented in the literature. Distance to a reconstructive surgeon has recently been shown to negatively impact BR rates. Roughton and associates found that greater than a 20-mile travel distance was a negative predictor of BR.32 While our study did not include travel distance as a covariate, many patients from rural Kentucky travel distances much greater than that in order to receive their breast cancer care, including BR. Collectively, the lack of access to a reconstructive surgeon and travel distance may be responsible in part for the disparity in BR between rural and urban patients in our study. Interventions focused on increasing BR surgeon density, referrals, and education could aid in resolving some of the disparities seen between rural and urban patients.
Breast cancer treatment including BR is a multidisciplinary process.33 Spyrou and colleagues showed the breast surgeons and surgical oncologists have negative views towards BR.34 Unfortunately, much of this is a result of dogma taught to surgeons who underwent training decades ago. At that time, it was thought that BR, whether autologous tissue or implant-based, could interfere with tumor surveillance. While this idea has since been refuted, clinical practice has been slow to accept this. Referral patterns to a reconstructive surgeon have also been shown as a barrier to BR. Patient referrals to a reconstructive surgeon may come from a rural community surgeon, a community surgeon in an urban environment, or within a comprehensive breast cancer care center. For the surgeon in the rural community, availability of a reconstructive surgeon is often a limiting factor. When no reconstruction is available, many times the community surgeon is responsible for educating patients on BR options and referring to a reconstructive plastic surgeon if the patient desires. Studies of referral patterns have shown that general surgeons who frequently involve reconstructive surgeons prior to mastectomy typically work in high-volume breast centers, while other general surgeons who do not involve reconstruction surgeons perceive higher patient barriers (ie, geography, cost).33 Furthermore, the timeline of referral to a reconstructive surgeon is also important, as it has been shown that patients are more likely to undergo BR if they receive education and undergo BR consultation pre-operatively.
BR is ultimately a patient decision. Interestingly, Lee and associates found that women are not well-informed about their BR options following mastecomty.35 Morrow and associates evaluated patients undergoing treatment for breast cancer and factors influencing their decisions for BR.36 They showed that patient decision tools, which include information about BR options, breast implant safety, and the impact of reconstruction on follow-up surveillance, address many patient-identified barriers to BR. Yet, while identifying modifiable factors to increase education and access to BR, there will always remain a cohort of patients who desire to limit their surgical procedures and decline reconstructive procedures. Nevertheless, patients must first be given the option of BR; the lack of a choice in the matter greatly threatens patient beneficence and autonomy, which are foundational principles of medical ethics.
Limitations
This study has several limitations that should be considered when interpreting results. First, the retrospective design limited the breadth of variables that could be examined. For example, we were unable to examine reconstruction type (implant vs. autologous) or the timing of BR (immediate vs. delayed). As such, it is possible that some patients underwent delayed reconstruction after the final date of data collection, which could have affected the reconstruction rates in our sample. Secondly, it did not take into account socioeconomic factors which have also been associated with BR. Additionally, our aggregated data represent BR in a single state. As a result, the rural-urban differences observed in this study may not be generalizable to other areas of the country, particularly those with differing patient demographics. This study did not examine the effect of Appalachian status on BR across the continuum. Additionally, the present study did not investigate the density or geographic distribution of reconstructive surgeons, which has also been shown to be predictive of BR.37 Another limitation was that the cancer registry was unable to provide the location where the mastectomy and/or BR was performed; this would have provided much more information regarding practice patterns. The ASPS database only identifies US board-certified plastic surgeons. As such, there may be other reconstructive surgeons offering reconstructive breast services that are either not board-certified or not members of ASPS. Finally, this study did not examine the relative importance that women across the rural-urban continuum place on BR, which could have affected study results.
Conclusion
Despite the increasing number of women diagnosed with breast cancer and undergoing mastectomy, and despite its benefits, rates of women undergoing BR remain relatively low. This study found a disparity in the utilization of BR in women from rural areas of Kentucky, which is significantly lower than what has been reported in the literature for patients from other rural areas. Breast cancer patients in urban and near-metro areas were more likely to undergo BR compared to their rural counterparts. Future research should seek to identify potential barriers to BR in this patient population. Finally, efforts should also seek to promote equitable access to BR for all patients, including those from rural areas.
Acknowledgements:
Data used in this publication were provided by the Kentucky Cancer Registry (KCR), Lexington, Kentucky. The authors would like to thank Jaclyn McDowell, DrPH, at KCR for assistance with data abstraction.
Funding: Drs. DeCoster and Bautista are supported by a National Institutes of Health (NIH), National Cancer Institute (T32CA160003): Oncology Research Training for Surgeon-Scientists training grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors have no associations or financial disclosures to report that create a conflict of interest with the information presented in this article.
References
- 1.Centers for Disease Control and Prevention. Breast Cancer Statistics. https://www.cdc.gov/cancer/breast/statistics/index.htm. Published 2019. Accessed July 15, 2019.
- 2.McCarthy CM, Hamill JB, Kim HM, Qi J, Wilkins E, Pusic AL. Impact of Bilateral Prophylactic Mastectomy and Immediate Reconstruction on Health-Related Quality of Life in Women at High Risk for Breast Carcinoma: Results of the Mastectomy Reconstruction Outcomes Consortium Study. Ann Surg Oncol. 2017;24(9):2502–2508. doi: 10.1245/s10434-017-5915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt JL, Wetzel CM, Lange KW, Heine N, Ortmann O. Patients’ experience of breast reconstruction after mastectomy and its influence on postoperative satisfaction. Arch Gynecol Obs. 2017;296(4):827–834. doi: 10.1007/s00404-017-4495-5 [DOI] [PubMed] [Google Scholar]
- 4.Kuroda F, Urban C, Zucca-Matthes G, et al. Evaluation of Aesthetic and Quality-of-Life Results after Immediate Breast Reconstruction with Definitive Form-Stable Anatomical Implants. Plast Reconstr Surg. 2016;137(2):278e–286e. doi: 10.1097/01.prs.0000475746.17968.f4 [DOI] [PubMed] [Google Scholar]
- 5.Sgarzani R, Negosanti L, Morselli PG, Vietti Michelina V, Lapalorcia LM, Cipriani R. Patient Satisfaction and Quality of Life in DIEAP Flap versus Implant Breast Reconstruction. Surg Res Pr. 2015;2015:1–7. doi: 10.1155/2015/405163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losken A, Jurkiewicz MJ. History of breast reconstruction. Breast Dis. 2002:3–9. doi: 10.3233/BD-2002-16102 [DOI] [PubMed] [Google Scholar]
- 7.Serletti JM, Fosnot J, Nelson JA, Disa JJ, Bucky LP. Breast reconstruction after breast cancer. Plast Reconstr Surg. 2011;127(6):124e–135e. doi: 10.1097/PRS.0b013e318213a2e6 [DOI] [PubMed] [Google Scholar]
- 8.Hu E, Alderman AK. Breast reconstruction. Surg Clin North Am. 2007;87:453–467. doi: 10.1016/j.suc.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 9.Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: Two-year postoperative results from the Michigan breast reconstruction outcomes study. Ann Surg. 2008;247(6):1019–1028. doi: 10.1097/SLA.0b013e3181728a5c [DOI] [PubMed] [Google Scholar]
- 10.Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: One-year postoperative results from the Michigan breast reconstruction outcome study. Plast Reconstr Surg. 2000;106(5):1014–1025. doi: 10.1097/00006534-200010000-00010 [DOI] [PubMed] [Google Scholar]
- 11.Women’s Health and Cancer Rights Act (WHCRA). https://www.cms.gov/cciio/programs-and-initiatives/other-insurance-protections/whcra_factsheet.html. Published 1998. Accessed July 9, 2019.
- 12.H.R.2540 - Breast Cancer Patient Education Act of 2015. https://www.congress.gov/bill/114th-congress/house-bill/2540/text. Published 2015. Accessed July 9, 2019.
- 13.Liu D New plastic surgery statistics and breast reconstruction trends | ASPS. New Plastic Surgery Statistics and Breast Reconstruction Trends. https://www.plasticsurgery.org/news/blog/new-plastic-surgery-statistics-and-breast-reconstruction-trends. Published 2018. Accessed June 4, 2018.
- 14.Tseng WH, Stevenson TR, Canter RJ, et al. Sacramento area breast cancer epidemiology study: use of postmastectomy breast reconstruction along the rural-to-urban continuum. Plast Reconstr Surg. 2010;126(6):1815–1824. doi: 10.1097/PRS.0b013e3181f444bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health Resources and Services Administration. Health Professional Shortage Areas. https://data.hrsa.gov/tools/shortage-area/hpsa-find. Published 2019. Accessed July 17, 2019.
- 16.Commission AR. Counties in Appalachia. https://www.arc.gov/appalachian_region/CountiesinAppalachia.asp. Published 2019. Accessed July 15, 2019.
- 17.Kamali P, Zettervall SL, Wu W, et al. Differences in the Reporting of Racial and Socioeconomic Disparities among Three Large National Databases for Breast Reconstruction. Plast Reconstr Surg. 2017;139(4):795–807. doi: 10.1097/PRS.0000000000003207 [DOI] [PubMed] [Google Scholar]
- 18.Connors SK, Goodman MS, Myckatyn T, Margenthaler J, Gehlert S. Breast reconstruction after mastectomy at a comprehensive cancer center. Springerplus. 2016;5(1):955. doi: 10.1186/s40064-016-2375-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong T, Hu J, Bagher S, et al. A Comparison of Psychological Response, Body Image, Sexuality, and Quality of Life between Immediate and Delayed Autologous Tissue Breast Reconstruction: A Prospective Long-Term Outcome Study. Plast Reconstr Surg. 2016;138(4):772–780. doi: 10.1097/PRS.0000000000002536 [DOI] [PubMed] [Google Scholar]
- 20.Sharma K, Grant D, Parikh R, Myckatyn T. Race and breast cancer reconstruction: Is there a health care disparity? Plast Reconstr Surg. 2016;138(2):354–361. doi: 10.1097/PRS.0000000000002344 [DOI] [PubMed] [Google Scholar]
- 21.Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Women’s Heal Issues. 2014;24(3):e261–e269. doi: 10.1016/j.whi.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offodile AC, Tsai TC, Wenger JB, Guo L. Racial disparities in the type of postmastectomy reconstruction chosen. J Surg Res. 2015;195(1):368–376. doi: 10.1016/j.jss.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 23.Wolfswinkel EM, Lopez SN, Weathers WM, et al. Predictors of post-mastectomy reconstruction in an underserved population. J Plast Reconstr Aesthet Surg. 2013;66(6):763–769. doi: 10.1016/j.bjps.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 24.Onega T, Weiss J, Kerlikowske K, et al. The influence of race/ethnicity and place of service on breast reconstruction for Medicare beneficiaries with mastectomy. Springerplus. 2014;3:416. doi: 10.1186/2193-1801-3-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng JF, Kronowitz SJ, Sun CC, et al. The effect of ethnicity on immediate reconstruction rates after mastectomy for breast cancer. Cancer. 2004;101(7):1514–1523. doi: 10.1002/cncr.20529 [DOI] [PubMed] [Google Scholar]
- 26.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: A study of the National Comprehensive Cancer Network. Ann Surg. 2006;243(2):241–249. doi: 10.1097/01.sla.0000197738.63512.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang JE, Summers DE, Cui H, et al. Trends in post-mastectomy reconstruction: A SEER database analysis. J Surg Oncol. 2013;108(3):163–168. doi: 10.1002/jso.23365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmoudi E, Giladi AM, Wu L, Chung KC. Effect of Federal and State Policy Changes on Racial/Ethnic Variation in Immediate Postmastectomy Breast Reconstruction. Plast Reconstr Surg. 2015;135(5):1285–1294. doi: 10.1097/PRS.0000000000001149 [DOI] [PubMed] [Google Scholar]
- 29.Health and Human Services. Environmental scan to identify the major research questions and metrics for monitoring the effects of the Affordable Care Act on safety net hospitals. C. Definition of safety net hospitals. https://aspe.hhs.gov/report/environmental-scan-identify-major-research-questions-and-metrics-monitoring-effects-affordable-care-act-safety-net-hospitals/c-definition-safety-net-hospitals. Published 2013. Accessed July 17, 2019.
- 30.Ballard TNS, Zhong L, Momoh AO, Chung KC, Waljee JF. Improved Rates of Immediate Breast Reconstruction at Safety Net Hospitals. Plast Reconstr Surg. 2017;140(1):1–10. doi: 10.1097/PRS.0000000000003412 [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute. NCI-Designated Cancer Centers. https://www.cancer.gov/research/nci-role/cancer-centers. Published 2019. Accessed July 17, 2019.
- 32.Roughton MC, DiEgidio P, Zhou L, Stitzenberg K, Meyer AM. Distance to a Plastic Surgeon and Type of Insurance Plan Are Independently Predictive of Postmastectomy Breast Reconstruction. 2017;138(2):1–17. doi: 10.1097/PRS.0000000000002343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alderman AK, Hawley ST, Waljee J, Morrow M, Katz SJ. Correlates of referral practices of general surgeons to plastic surgeons for mastectomy reconstruction. Cancer. 2007;109(9):1715–1720. doi: 10.1002/cncr.22598 [DOI] [PubMed] [Google Scholar]
- 34.Spyrou GE, Titley OG, Cerqueiro J, Fatah MF. A survey of general surgeons’ attitudes towards breast reconstruction after mastectomy. Ann R Coll Surg Engl. 1998;80(3):178–183. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CN, Belkora J, Chang Y, Moy B, Partridge A, Sepucha K. Are patients making high-quality decisions about breast reconstruction after mastectomy? Plast Reconstr Surg. 2011;127(1):18–26. doi: 10.1097/PRS.0b013e3181f958de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow M, Li Y, Alderman AK, et al. Access to breast reconstruction after mastectomy and patient perspectives on reconstruction decision making. JAMA Surg. 2014;149(10):1015–1021. doi: 10.1001/jamasurg.2014.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauder AR, Cary P, Killelea BK, Butler PD, Kovach SJ, Justin P. The Relationship Between Geographic Access to Plastic Surgeons and Breast Reconstruction Rates Among Women Undergoing Mastectomy for Cancer. 2017;78(3):324–329. doi: 10.1097/SAP.0000000000000849 [DOI] [PubMed] [Google Scholar]