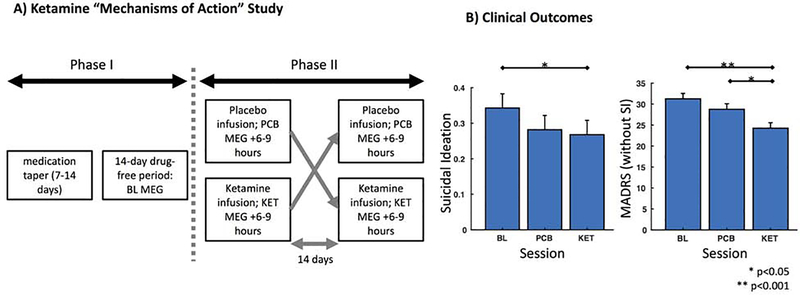
Figure 1. Study Design and Clinical Outcomes.
A) The study included a double-blind, placebo-controlled, crossover design. During Phase I, patients tapered off their medications and completed a 14-day drug-free period prior to entering Phase II. A baseline (BL) MEG recording was collected during this time-period (specifically, 2 to 4 days prior to the first infusion). During Phase II, patients received both ketamine (KET) and placebo saline (PCB) infusions, with a 14-day period between crossover. MEG recordings were collected 6–9 hours following each infusion. B) Clinically, patients showed significant reductions in suicidal ideation and depression scores following ketamine administration compared to baseline. In addition, patients showed significant reductions in depression scores between the ketamine and placebo sessions.