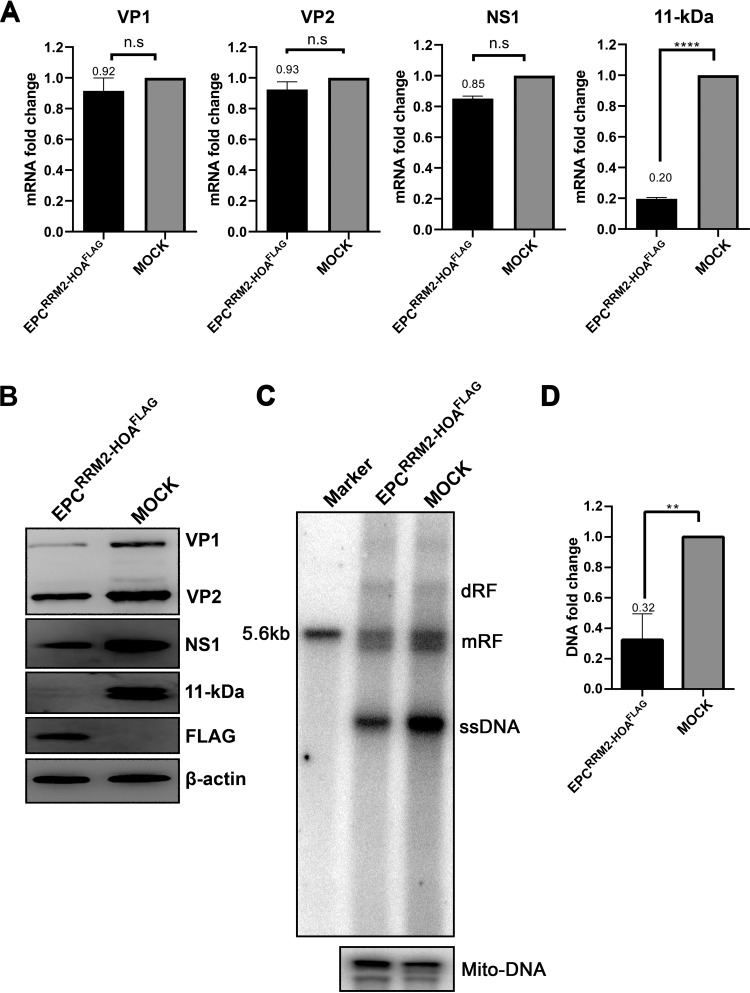
FIG 7.
Expression of the ISE3-binding domain of RBM45 decreased expression of 11-kDa at the levels of both mRNA and protein. CD36+ EPCs were transduced with a lentivirus expressing RBM45-RRM2-HOA. At 3 days postransduction, the cells were infected with B19V. After 48 h, the cells were collected for analyses of expression of viral mRNAs and proteins and of viral DNA replication. (A) RT-qPCR. Total RNA was extracted from CD36+ EPCs and reverse transcribed to generate cDNA, which was used for quantification of the viral mRNAs that encode the VP1, VP2, NS1, and 11-kDa proteins by the use of qPCR. The quantified viral mRNA levels were normalized to the level of β-actin mRNA, and the values representing mRNAs quantified from B19V-infected control CD36+ EPCs were arbitrarily set at 1. (B) Western blotting. The cells were lysed and analyzed by Western blotting. The blots were probed for the VP1/2, NS1, and 11-kDa proteins, using their respective antibodies. β-Actin was reprobed as a loading control. (C and D) Southern blotting. At 2 days posttransfection, Hirt DNA samples were prepared from CD36+ EPCs and were subjected to Southern blot analysis. SalI-digested pM20 (10 ng) was loaded as a size marker. (C) The blots were probed with the M20 probe (top) and the Mito-DNA probe (bottom). Representative blots are shown. dRF, mRF, and ssDNA, double replicative form, monomer replicative form, and single-stranded DNA, respectively. (D) The intensity of the RF DNA band was quantified and normalized to the level of the mitochondrial DNA (Mito-DNA) of each sample. The value representing viral RF DNA in B19V-infected CD36+ EPCs transduced with shScram was arbitrarily set to 1. Relative fold change of the viral RF DNA in B19V-infected CD36+ EPCs transduced with shRBM45 is shown with averages and standard deviations of results from three repeated experiments.