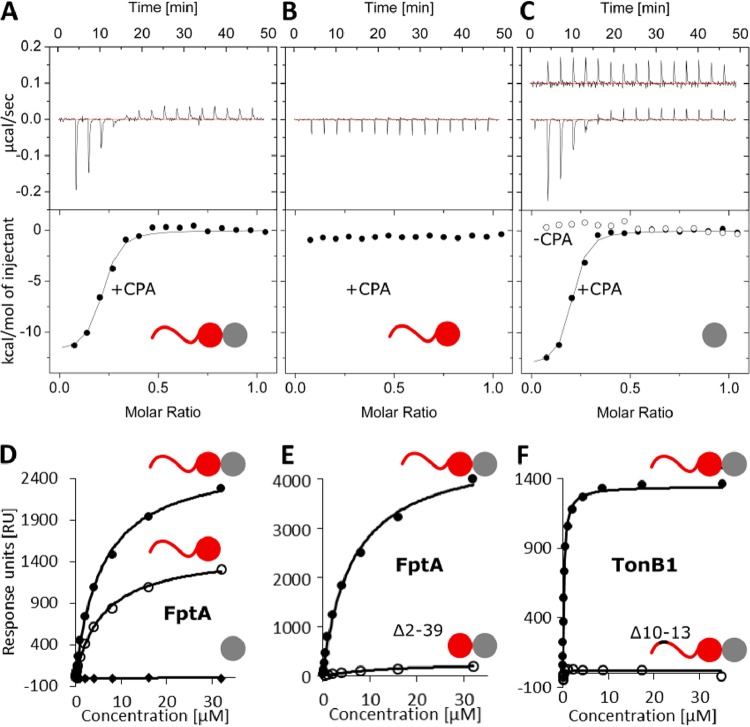
FIG 2.
kTHB domain 2 binds CPA, kTHB domain 1 binds FptA, and the N-terminal disordered region binds TonB1. (A and B) ITC data for PyoS51–315 titrated into P. aeruginosa PAO1 LPS-derived polysaccharide containing CPA and OSA (closed circles) gives a Kd of 612 ± 332 nM (A), and PyoS51–196 titrated into P. aeruginosa PAO1 LPS-derived polysaccharide shows no binding (B). (C) ITC data for PyoS5194–315 titrated into P. aeruginosa PAO1 LPS-derived polysaccharide gives a Kd of 269 ± 44 nM. PyoS5194–315 titrated into P. aeruginosa Δrmd LPS-derived polysaccharide containing OSA only (open circles) shows no binding. (A to C) Kd values and concentrations can be found in Table S2B. All ITC experiments were performed in duplicate in 0.2 M Na-phosphate buffer (pH 7.5) at 25°C, and one repeat is shown. Data were corrected for heats of dilution by subtracting the average of the last five injections and fit to a model of single-site binding. (D) SPR data for FptA (0.03 to 32 μM) binding to PyoS51–315 (closed circles; Kd, 6.5 ± 0.4 μM), PyoS51–196 (open circles; Kd, 7.1 ± 0.7 μM), or PyoS5194–315 (diamonds; no binding). PyoS51–315 achieves higher RU levels than does PyoS51–196 even though the binding affinity remains essentially unchanged. Since the pyocin was immobilized randomly on the chip, this is likely due to the greater availability of FptA binding sites in the larger (PyoS51–315) construct. (E) SPR data for FptA (0.03 to 32 μM) binding to PyoS51–315 (closed circles; Kd, 6.5 ± 0.4 μM) or PyoS51–315 Δ2–39 (open circles; Kd, 14.7 ± 0.4 μM). (F) SPR data for TonB1 (0.009 to 35 μM) binding to PyoS51–315 (closed circles; Kd, 241 ± 9 nM) or PyoS51–315 Δ10–13 (open circles; no binding). (D to F) One of three repeats is shown. All experiments were performed in parallel on the same chip in HBS-OG buffer at 25°C. All ligands were immobilized by amine coupling, and sensorgram data were extracted and fit with a 1:1 binding model. Kd values are presented in Table S2C.