FIG 2.
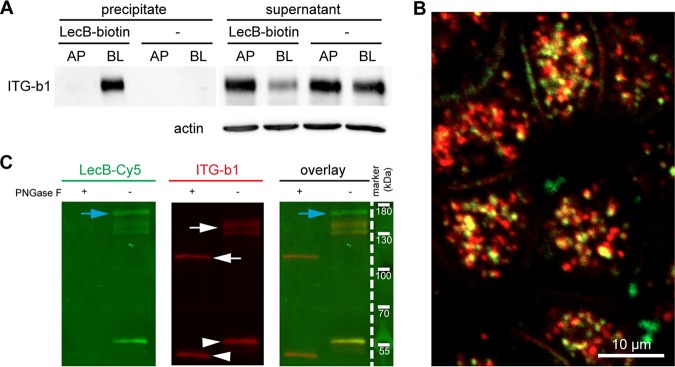
LecB directly binds to β1-integrin. (A) LecB-biotin was applied apically (AP) or basolaterally (BL) to polarized filter-grown MDCK cells, or cells were mock treated AP or BL. Cells were lysed, and LecB-biotin-receptor complexes were precipitated with streptavidin beads. Afterward, the presence of β1-integrin was probed by Western blotting in the precipitate and the remaining supernatant of the precipitation. (B) LecB-Cy3 (red) was applied basolaterally to MDCK cells for 6 h. Cells were fixed and stained for β1-integrin (green). A confocal section (x-y section) crossing the cells in the subapical region is displayed, since most internalized vesicles were concentrated in this region. (C) MDCK cells were lysed, and β1-integrins were immunoprecipitated and treated or left untreated with peptide-N-glycosidase F (PNGase F) to remove N-linked glycans. Western blot analysis of the immunoprecipitated β1-integrins was performed, and β1-integrin presence was proven by staining with anti-β1-integrin antibodies (white arrows). Also, bands from the antibody used for β1-integrin precipitation (white arrowheads) and proteins that putatively coprecipitated with β1-integrin (blue arrows) are visible. To probe the binding of LecB to β1-integrin, LecB-Cy5 was incubated with membranes (far-Western assay).