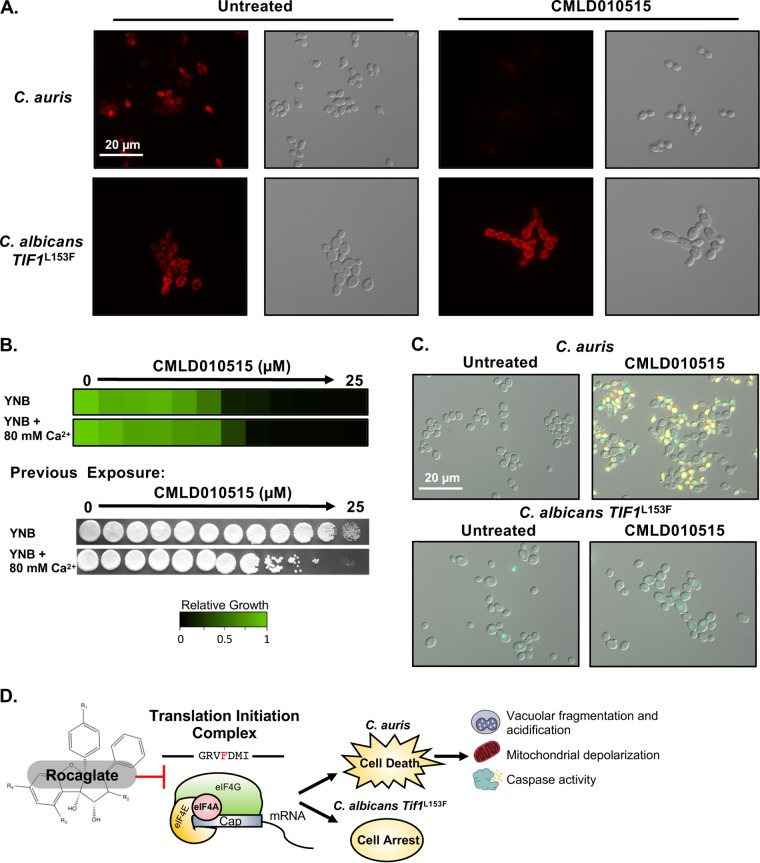
FIG 5.
Sensitive C. auris cells display a loss of mitochondrial membrane potential and increased caspase activity. (A) Loss of mitochondrial membrane potential was visualized by the use of MitoTracker Red. Cells were subcultured and then treated with 50 μM CMLD010515 for 3 h at 30°C with agitation. MitoTracker Red was added to the culture at 50 nM, and the cells were further incubated for 1 h. Cells were imaged by differential interference contrast microscopy and the use of the DsRed channel on a Zeiss Axio Imager.MI microscope, with the exposure time remaining constant between samples. (B) The sensitivity of C. auris to the rocaglates in YNB medium containing low calcium levels (0.68 mM) or excess calcium (80 mM) was assessed by dose-response assays, as described for Fig. 1 (top panel). Cells were taken from the MIC assays, and 5 μl was spotted onto YPD medium without drug. Plates were incubated at 30°C for 24 h and imaged (bottom panel). (C) To detect caspase activity, a CaspSCREEN assay kit was used. Cells were subcultured for 18 h in the absence or presence of 50 μM CMLD010515. Cells were resuspended in buffer containing the caspase substrate for 45 min. Propidium iodide was added at 1 μg/ml to visualize dead cells. Cells were imaged on the EGFP channel for caspase activity (green) and on the DsRed channel for death (yellow) and by differential interference contrast microscopy on a Zeiss Axio Imager.MI microscope, all at constant exposure. (D) The rocaglates inhibit translation initiation complex member eIF4A, and the inhibition is contingent on the presence of specific residues in the drug binding pocket. The inhibition leads to cell death in C. auris, in which phenotypes such as vacuolar hyperacidification and fragmentation, mitochondrial depolarization, and increased caspase-like activity are observed. In contrast, rocaglate treatment results in cell arrest in C. albicans in the absence of features of cell death programs.