Figure 3.
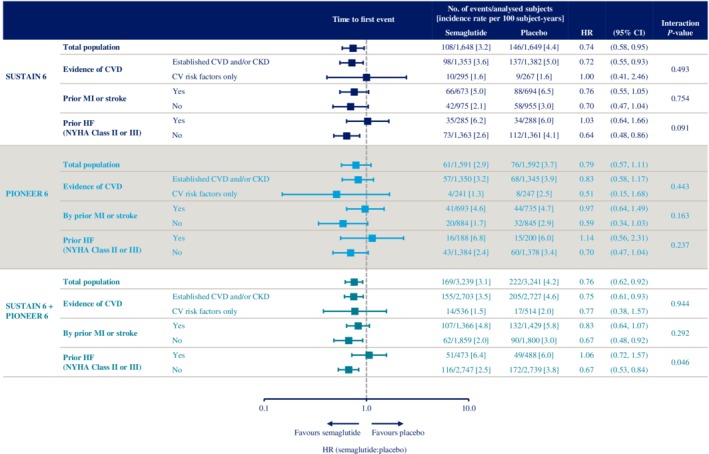
Time to first occurrence of event adjudication committee‐confirmed three‐component major adverse cardiovascular events (MACE) (cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) with semaglutide versus placebo in SUSTAIN 6 and PIONEER 6 CV risk subgroups. SUSTAIN 6 and PIONEER 6 have already been individually reported.32, 33 Data are “in‐trial”, analysed using Cox proportional hazards models with treatment and subgroup as categorical fixed factors, with interaction term between treatment and subgroup, stratified by trial and CV risk at screening. P‐value: unadjusted interaction test between treatment and subgroups. CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; NYHA, New York Heart Association
