Figure 4.
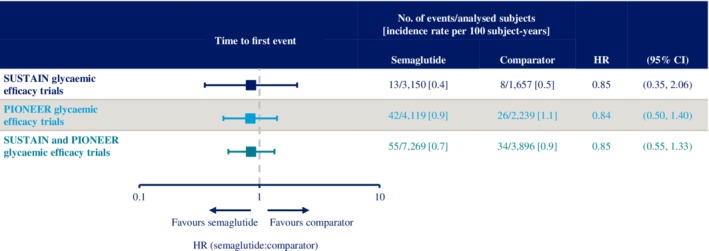
Time to first occurrence of major adverse cardiovascular events (MACE) (cardiovascular [CV] death, nonfatal myocardial infarction [MI] and nonfatal stroke) across the SUSTAIN and PIONEER phase 3a glycaemic efficacy trials. Data are “in‐trial” from SUSTAIN 1–5 and two SUSTAIN JAPAN trials, and PIONEER 1–5, 7–8 and the two PIONEER Japanese trials (9 and 10). Times from randomization to first event adjudication committee‐confirmed MACE analysed using a stratified Cox proportional hazards model with treatment as a categorical fixed factor and stratified by trial. See Tables S2 and S3 for details of comparators. CI, confidence interval; HR, hazard ratio
