Figure 2.
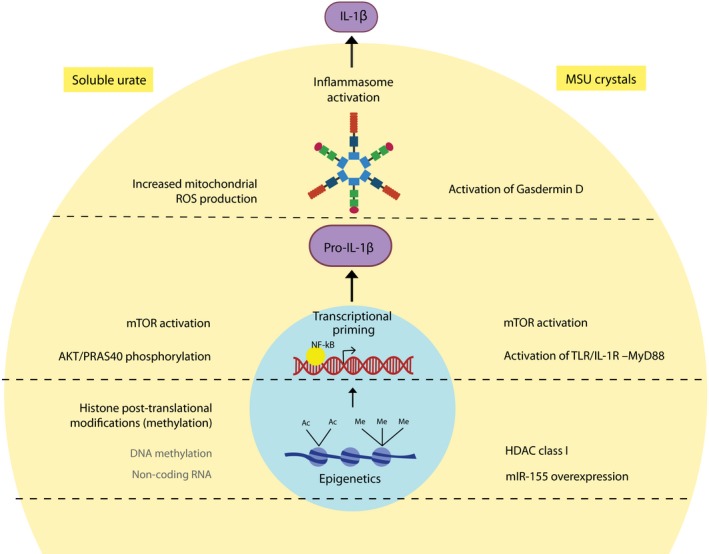
Mechanisms of soluble urate– and monosodium urate crystal–induced inflammation. Soluble urate and MSU crystals promote inflammation at several different levels. Inflammasome activation: MSU crystals activate the NLRP3 inflammasome and are accompanied by activation of gasdermin D. Soluble urate can also activate the NLRP3 inflammasome and seems to be dependent on the production of mitochondrial ROS. Transcriptional control: MSU crystals induce mTOR activation and inhibition of PTEN (an mTOR inhibitor) which further induce IL‐1β and inhibition of mTOR leads to lower IL‐1β production. MSU crystals act through MyD‐88, an intracellular adapter protein that leads to activation of NF‐κB and IL‐1β expression. Soluble urate priming enhanced PRAS40 phosphorylation which activates mTOR through Akt signaling leading to IL‐1β expression. Epigenetic regulations: MSU crystal stimulation of innate immune cells is associated with class I HDAC activation accompanied by SOCS1 downregulation, NF‐κB activation, and enhanced IL‐1β transcription. MSU crystals also induce the expression of miR‐155 which by inhibiting SHIP‐1 leads to phosphorylation of Akt with subsequent activation of NF‐κB and IL‐1β transcription. Soluble urate priming is regulated epigenetically by histone methylation, perhaps also by involvement of DNA methylation and non‐coding RNAs, with inhibition of histone methyltransferase opposing the effects of urate. MSU: monosodium urate; HDAC: histone deacetylase; SOCS1: suppressor of cytokine signaling 1; NF‐κB: nuclear factor kappa B; IL: interleukin; miR: microRNA; Akt: protein kinase B; SHIP‐1: Src homology 2 (SH2) domain‐containing inositol polyphosphate 5‐phosphatase 1; PTEN: phosphatase and tensin homolog; mTOR: mammalian target of rapamycin; MyD‐88: myeloid differentiation primary response 88; PRAS40: proline‐rich Akt substrate of 40 kDa; NLRP3: NACHT, LRR, and PYD domain‐containing protein 3; ROS: reactive oxygen species
