Scheme 2.
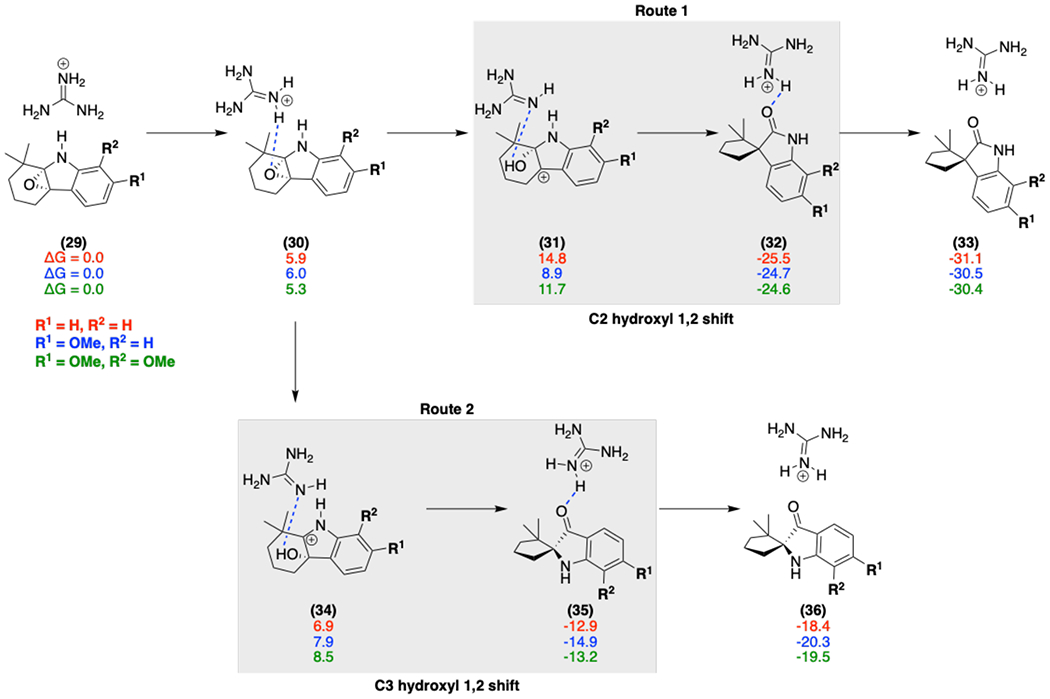
Free energies (calculated at the M06-2X/6-311++G(d,p)-ECFPCM(water)//M06-2X/6-31G(d)-IEFPCM (water) level) for various models (red, blue, green) with the Arg192 general acid (modeled by a guanidinium moiety) catalyzed epoxide ring-opening mechanism; initial protonation of the epoxide leads to ring opening to form a hydroxy cation (31 and 34), and deprotonation accompanies the 1,2 shift to generate the final spirocycle product.
