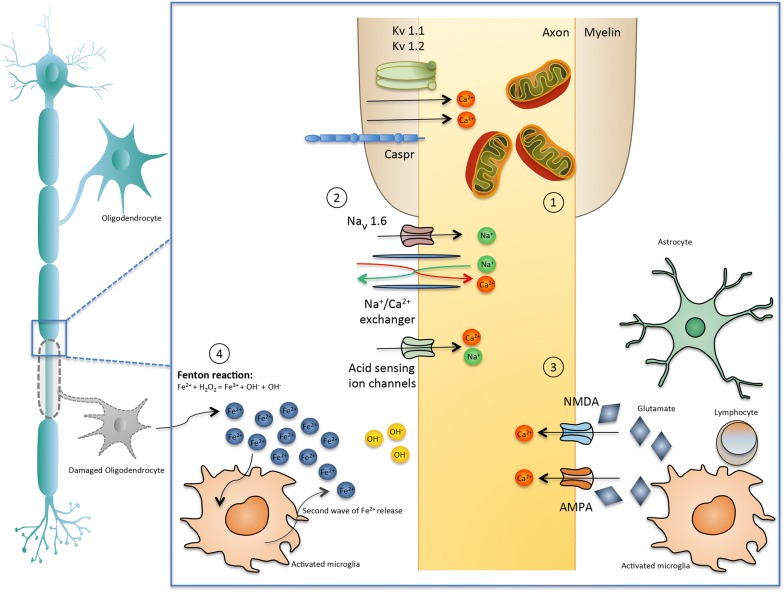
Fig. 1.
Mechanisms of late axonal loss. Molecular and cellular mechanisms driving neurodegeneration and atrophy. Key elements are considered to be: (1) Mitochondria Dysfunction: Inflammation in acute demyelinating lesions lead to respiratory protein complexes inhibition, mitochondrial injury and dysfunction, release of apoptosis-inducing factors and mitochondrial DNA deletions. In chronic inactive plaques, ionic imbalance, high energy demands and clonal expansion of defective mitochondria further impair oxidative damage. These mitochondrial alterations of functional impairment and structural damage lead to histotoxic hypoxia and energy failure and consequently to neurodegeneration. [146] Upregulation of sodium channels, acid sensing ion channels and expression of maladaptive isoforms (Nav1.6 channels), paranodal (Caspr) and juxtparanodal (Kv1.2) protein lead to high energy demands, intra-axonal calcium accumulation, and subsequent axonal degeneration. (3) Glutamate Excitotoxicity: Increased glutamate production by activated microglial cells and lymphocytes, and impaired clearance by resident cells such as astrocytes lead to higher lever of glutamate. High levels of glutamate lead to over-activation of N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (which are permeable for calcium and sodium ions) and subsequent calcium overload and oligodendocyte and neuron cell death. (4) Iron release: In MS lesions free iron [Fe2+] is released in the extracellular space leading to production of highly reactive hydroxyl molecules (OH−) by the Fenton reaction. Further, iron is released by activated glial cells, which become dystrophic and disintegrate, leading to a second wave of Fe2+ release