Abstract
Although a ‘vascular stem cell’ population has not been identified or generated, vascular endothelial and mural cells (smooth muscle cells and pericytes) can be derived from currently known pluripotent stem cell sources including human embryonic stem cells and induced pluripotent stem cells. We review the vascular potential of these human pluripotent stem cells, the mechanisms by which they are induced to differentiate toward a vascular endothelial cell fate, and their applications in regenerative medicine.
Key Words: Angiogenesis, Blood vessel biology, Endothelial cell differentiation, Regenerative medicine, Stem cell differentiation
Introduction
Stem cells are totipotent or pluripotent cells capable of self-renewal and asymmetric division, which can give rise to multiple types of specialized or differentiated cells. These cells have been derived from the inner cell mass of mammalian embryos including mice, rats, and humans [Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998; Buehr et al., 2008; Li et al., 2008], from a variety of postnatal organs [Altman, 1969; Goldman and Nottebohm, 1983; Morrison and Weissman, 1994; Rochat et al., 1994; Lagasse et al., 2001], and from the ‘reprogramming’ of somatic cells [Takahashi et al., 2007; Yu et al., 2007]. Collectively, such stem cells are seen as potentially infinite resources from which all cell types of the body can be derived. The study of their development, differentiation, and function is therefore central to the potential of regenerative medicine.
| Abbreviations used in this paper | |
|---|---|
| bFGF | basic fibroblast growth factor |
| EB | embryoid body |
| ES | embryonic stem |
| HDAC | histone deacetylase |
| hES | human embryonic stem |
| HIF | hypoxia-inducible factor |
| hiPS | human induced pluripotent stem |
| Ihh | Indian hedgehog |
| iPS | induced pluripotent stem |
The broad field of regenerative medicine seeks to channel knowledge of the molecular and cellular mechanisms by which specific cell and tissue types are derived into the development of clinical therapies for tissue repair/replacement. Regenerative medicine strategies utilize a noninclusive combination of cells, scaffolds, and bioactive factors to replace or restore function to failing or injured tissues. Progress in the field has been reviewed broadly [Gurtner et al., 2007] and with respect to the utilization of stem or progenitor cells [Blau et al., 2001; Amabile and Meissner, 2009], the utility of natural and synthetic scaffolds [Lutolf and Hubbell, 2005; Badylak, 2007], and controlled presentation and release of bioactive molecules [Putnam and Mooney, 1996; Shin et al., 2003].
While the nascent field continues to progress, the greatest obstacle to further advancement continues to be challenges associated with vascularization of engineered constructs. Nonetheless, substantial regenerative medicine successes have been accomplished via transplantation of vascular grafts [Campbell et al., 1999; Niklason et al., 1999], decellularized tissues [Badylak et al., 2010; Quint et al., 2011] and engineered tissues that did not require in vitro vascularization [Atala et al., 2006; Nakahara and Ide, 2007]. For the regenerative medicine field to realize its full potential, however, a dependable source of vascular cells must be identified, and our ability to control the differentiation and specialization of such vascular cells must be improved.
To date, a ‘vascular stem cell’ population has not been identified or generated. However, vascular endothelial and mural cells (smooth muscle cells and pericytes) can be derived from currently known pluripotent stem cell sources including human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells. Additionally, vascular cells have been derived from progenitor cells isolated from human bone marrow, peripheral blood, adipose tissue, skeletal muscle, and various vascular beds [Castro-Malaspina et al., 1980; Galmiche et al., 1993; Asahara et al., 1997; Kalka et al., 2000; Murohara et al., 2000; Zuk et al., 2001; Majka et al., 2003; Crisan et al., 2008]. Although there is controversy about the exact phenotype(s) of vascular progenitor cells, they are generally thought to function as immediate precursors to vascular endothelial and/or mural cells, with a limited capacity to generate other lineages. The phenotype and function of adult vascular progenitor/precursor cells have been extensively reviewed elsewhere [Hirschi et al., 2008]; this review will focus on the vascular potential of human pluripotent stem cells and the mechanisms by which they are induced to differentiate toward a vascular endothelial cell phenotype.
Human ES Cell-Derived Vascular Cells
In 1998, Thomson et al. [1998] were the first group to report successful isolation of human ES (hES) cells. Since then, numerous groups have demonstrated the potential of hES cells to differentiate into various cell types originating from all three germ layers. For this review, we will focus specifically on the potential of hES cells to give rise to vascular endothelial cells that form the luminal layer of blood vessels. The potential of human stem and progenitor cells to give rise to mural cells that form the surrounding vessel wall is addressed in other reviews in this miniseries.
Vascular endothelial cell differentiation is induced in hES cells via two commonly used methods, i.e. embryoid body (EB) formation [Levenberg et al., 2002] and coculture on monolayers of OP9 cells (murine bone marrow stromal cells) [Vodyanik et al., 2005; Kelly and Hirschi, 2009]. In the EB formation approach, hES cells spontaneously differentiate into cell types representing all three germ layers. Cells expressing surface markers consistent with primordial endothelial cells (i.e. CD31 and VE-cadherin) can then be isolated using flow cytometry and subcultured on growth factor-supplemented fibronectin, or other extracellular matrices, to promote endothelial cell proliferation [Levenberg et al., 2002; Gerecht-Nir et al., 2003].
The coculture of hES cells on OP9 feeder cells was first established to differentiate mouse ES cells into hematopoietic cells [Nakano et al., 1994] and was recently found to promote robust generation of vascular endothelial cells from hES cells [Kelly and Hirschi, 2009]. Perhaps this is not surprising given that hematopoietic stem/progenitor cells are derived from the endothelium during mouse and human embryonic development [for review, please see Sills and Hirschi, 2011]. Expression of endothelial-specific markers begins to appear between days 10 and 14 of coculture, followed by the upregulation of hematopoietic markers by day 21 [Kelly and Hirschi, 2009], demonstrating that coculture of hES cells on OP9 cells promotes sequential differentiation of endothelial and hematopoietic cells.
Although both of these culture systems are useful for generating human endothelial cells, there are drawbacks to both for studying the molecular regulation of this process, such as the presence of undefined serum and other cell types that may contribute to vascular cell differentiation. Thus, the field is moving toward more defined serum-free and feeder-free culture systems.
Mechanism of Differentiation of hES to Endothelial Cells
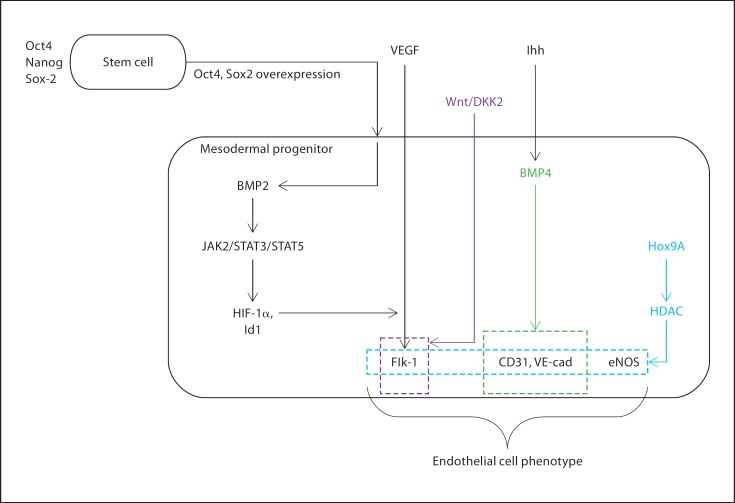
Multiple developmental model systems including avian, zebrafish, and mouse embryos have been used to understand the mechanisms involved in the regulation of endothelial cell development. We will review what is currently known about the differentiation of endothelial cells from pluripotent stem cells, predominantly human and mouse ES cells (overview in fig. 1). A transcriptional network involving Oct4/Nanog/Sox2 regulates ES cell pluripotency, and the overexpression of Oct4/Sox2 appears to promote the generation of mesodermal progenitors [Bosnali et al., 2009]. Several soluble factors localized at the site of murine vasculogenesis have been shown to play a functional role in the generation of endothelial cells, presumably from mesodermal progenitors, in vivo and in murine ES cells. Such regulators, including Indian hedgehog (Ihh) [Dyer et al., 2001], VEGF [Carmeliet et al., 1996], and basic fibroblast growth factor (bFGF) [Leconte et al., 1998], have also more recently been shown to regulate endothelial cell differentiation from hES cells [Kelly and Hirschi, 2009]. Coordinated signaling through the Ihh and bFGF pathways is thought to be necessary for the upregulation of VEGF receptor 2 (VEGFR2 or KDR or Flk-1). In the mouse, molecules of the Wnt signaling pathway are differentially regulated in Flk-1+ cells, compared with Flk-1– cells, suggesting a role for Wnt activity during vascular endothelial cell differentiation in rodents and humans [Min et al., 2011].
Fig. 1.
Schematic depicting the mechanisms of human endothelial cell differentiation.
Although we know that Ihh promotes endothelial cell differentiation from hES cells via BMP signaling [Kelly and Hirschi, 2009; Bai et al., 2010], less is known about pathways downstream of BMP in this system relative to the murine and rat models. For example, in the rat model, Belmokhtar et al. [2011] showed that recombinant human BMP2 enhances the expression of hypoxia-inducible factor (HIF)-1α and inhibitor of DNA binding gene (Id1) through the stimulation of JAK2/STAT3/STAT5 signaling pathways. It is known that HIF-1α is involved in the activation of VEGF transcription needed for endothelial cell propagation [Forsythe et al., 1996] and that HIF-1α-null mouse embryos have lethal vascular malformations [Semenza, 2000]. Although Ihh, BMP factors, and HIF-1α-induced VEGF are important for human vascular development, the intracellular signaling pathways downstream of these effectors, and their interactions, have yet to be determined.
Work from adult human endothelial progenitor cells also suggests that acetylation plays an important role in determining cell fate decisions and promotion of an endothelial phenotype. Endothelial cell commitment from circulating progenitor cells is thought to require histone deacetylase (HDAC) activity and to depend on the expression of the homeobox transcription factor HoxA9 [Rossig et al., 2005]. HoxA9 is essential to maintaining the expression of a variety of endothelial-specific genes such as eNOS, Flk-1, and VE-cadherin in cultured endothelial cells. Furthermore, HoxA9-deficient mice exhibit severe impairment in neovascularization and tissue recovery after hind limb ischemia, which may be secondary to the contribution of HoxA9-induced genes to endothelial cell monolayer recovery. Certainly, much remains to be elucidated about the regulation of human endothelial cell differentiation and function.
Human iPS Cell-Derived Vascular Cells
Human iPS (hiPS) cells are adult somatic cells that have been genetically reprogrammed to an ES cell-like state via forced expression of genes needed to maintain the defining properties of ES cell pluripotency. The process of generating hiPS cells initially began with the use of retroviruses and/or lentiviruses to transduce regulatory genes either separately or in a single expression vector [Takahashi et al., 2007; Yu et al., 2007; Carey et al., 2009]. The Thomson and Yamanaka labs independently were able to transduce into fibroblasts genes encoding transcriptional regulators of stem cells, i.e. Nanog, Lin28, Oct4, and Sox2 [Yu et al., 2007] or c-Myc, Klf4, Oct4, and Sox2 [Takahashi et al., 2007], to induce a pluripotent phenotype. However, there are disadvantages to using viruses to reprogram somatic cells with regard to clinical potential. For example, viruses integrate into host chromosomes, which may lead to insertional mutagenesis and interferences of gene transcription, resulting in malignant transformations. In fact, Yamanaka’s group found that 20% of mice injected with mouse iPS cells developed tumors attributed to reactivation of c-Myc proviral transgene [Okita et al., 2007]. Subsequently, many labs are developing alternate strategies that do not require viruses to reprogram differentiated cells into iPS cells, to allow for subsequent human therapy [Woltjen et al., 2009; Lee et al., 2011; Montserrat et al., 2011].
Reprogrammed hiPS cells are thought to be similar to hES cells in terms of morphology, proliferation status, surface antigen expression, the epigenetic status of pluripotent cell-specific genes, and telomerase activity. Furthermore, hiPS cells can differentiate into cell types of all three germ layers in vitro and form teratomas in vivo [Takahashi et al., 2007]. Several groups (i.e. Christodoulou et al. [2011]) have also reported similarities between the two cell types at a transcriptional level while pointing out some key differences between the profiles of these two cell types. In one example, global gene expression profiles of hiPS and hES cells were compared for 32,266 transcripts, and, although there was an overall similar global gene expression profile, approximately 4% of the transcriptionally profiled genes had a greater than 5-fold difference in expression level between hiPS and hES cells [Takahashi et al., 2007]. It is important to note that heterogeneity in gene expression levels also exists between different hiPS cell lines themselves [Narsinh et al., 2011]. Thus, although hiPS cells derived from various protocols have been differentiated into vascular and hematopoietic cell types similar to hES cells [Boheler, 2010; Feng et al., 2010], it is unclear whether the differences in gene expression among iPS cell lines, or between hiPS and ES cells, will have consequences on their overall vascular potential and/or the pathways that regulate their differentiation toward vascular cell fates. More work is needed to investigate the vascular potential, and possible vascular bias, of iPS cells derived from distinct somatic cell types and the mechanism(s) regulating their differentiation toward an endothelial cell fate.
Applications in Regenerative Medicine
The ability to differentiate hES cells [Levenberg et al., 2002], and more recently hiPS cells [Boheler, 2010], into endothelial cells provides additional resources to address a primary challenge in the field of tissue engineering: tissue vascularization. Access to human, nontransformed endothelial cells generates expanded research opportunities ranging from an improved understanding of human vascular development to a readily available cell source for regenerative medicine applications. To date, the body of literature concerning the use of vascular cells in combination with tissue-specific cells for regenerative medicine applications remains limited. However, recently, endothelial cells of various origins were incorporated into tissue-engineered constructs to promote myocardial regeneration [Tulloch et al., 2011], dermal tissue regeneration [Zhang et al., 2011], and skeletal muscle growth [Levenberg et al., 2005], which resulted in improved integration into the host. Tulloch et al. [2011] demonstrated that the addition of endothelial cells to the human cardiac cells resulted in an increase in lumen containing structures in vitro and a contribution to functional vasculature in vivo. The necessity of mural cell involvement for vessel maturation is well characterized [Jain, 2003] and the in vivo studies which utilized vascular support cells in their constructs saw enhanced engraftment and vascularization compared with endothelial cells alone [Levenberg et al., 2005; Tulloch et al., 2011]. Multiple groups have now shown the ability to derive smooth muscle cells from pluripotent stem cells, further demonstrating the potential of the hES and hiPS cell lines for vascular engineering applications [Ferreira et al., 2007], as reviewed elsewhere in this miniseries.
Successes in animal models will eventually translate into modern medicine leading to an increased demand for therapeutically appropriate human cells. The ability to derive any human cell population from pluripotent stem cells establishes this technology as a critical component in the future of medicine. As the regenerative medicine field progresses, design challenges concerning construct vascularization will remain a high priority. To optimize construct engraftment, from a vascular perspective, investigators must determine the optimal balance of transplanted cells versus recruited endogenous cells along with methods to facilitate recruitment. This challenge leads to questions concerning the degree of construct organization or in vitro maturation necessary for a construct to functionally integrate into the host. How elegant must our designs be and how do we design for in vivo remodeling? Additionally, we must determine the optimal state of differentiation for expansion of pluripotent stem cell-derived endothelial cells along with what state is ideal for incorporation into a tissue-engineered construct and host tissue. Our ability to derive vascular cells from pluripotent stem cells has been clearly demonstrated and as these techniques are refined, based on a better understanding of molecular regulation, they will certainly make impactful contributions to the progress of regenerative medicine.
In summary, it is important to note that human pluripotent stem cells have opened a huge window of cell therapies tailored to individual needs, which will eliminate the problems attached to transplant rejection. However, it is also vital to acknowledge that insights derived from studies of murine ES cells may not be directly applicable to human studies; thus, continued work in human pluripotent stem cell model systems is imperative for advancement in the field.
References
- Altman J, Autoradiographic and histological studies of postnatal neurogenesis 4. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–966. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Atala A, Bauer S. B., Soker S, Yoo J. J., Retik A. B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Badylak S. F., Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2010;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Gao Y, Arzigian M, Wojchowski D. M., Wu W. S., Wang Z.Z. BMP4 regulates vascular progenitor development in human embryonic stem cells through a smad-dependent pathway. J Cell Biochem. 2010;109:363–374. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmokhtar K, Bourguignon T, Worou M, Khamis G, Bonnet P, Domenech J, Eder V. Regeneration of three layers vascular vall by using BMP2-treated MSC involving HIF-1α and Id1 expressions through JAK/STAT pathways. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9254-6. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Blau H.M., Brazelton T.R., Weimann J.M. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- Boheler K.R. Pluripotency of human embryonic and induced pluripotent stem cells for cardiac and vascular regeneration. Thromb Haemost. 2010;104:23–29. doi: 10.1160/TH09-07-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnali M, Münst M, Thier B, Edenhofer F. Deciphering the stem cell machinery as a basis for understanding the molecular mechanism underlying reprogramming. Cell Mol Life Sci. 2009;66:3403–3420. doi: 10.1007/s00018-009-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying Q.L., Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Campbell J.H., Efendy J.L., Campbell G.R. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ Res. 1999;85:1173–1178. doi: 10.1161/01.res.85.12.1173. [DOI] [PubMed] [Google Scholar]
- Carey B.W., Markoulaki S, Hanna J, Saha K, Gao Q, Mitalipova M, Jaenisch R. Reprogramming of murine and human somatic cells using a single polycistronic vector. Proc Natl Acad Sci USA. 2009;106:157–162. doi: 10.1073/pnas.0811426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Castro-Malaspina H, Gay R. E., Resnick G. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- Christodoulou C, Longmire T. A., Shen S. S., Bourdon A, Sommer C.A., Gadue P, Spira A, Gouon-Evans V, Murphy G.J., Mostoslavsky G, Kotton D.N. Mouse ES and iPS cells can form similar definitive endoderm despite differences in imprinted genes. J Clin Invest. 2011;121:2313–2325. doi: 10.1172/JCI43853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen C. W., Corselli M, Park T. S., Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P. N., Traas J, Schugar R, Deasy B. M., Badylak S, Buhring H.J.r., Giacobino J. P., Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dyer M.A., Farrington S. M., Mohn D, Munday J. R., Baron M.H. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Feng Q, Lu S. J., Klimanskaya I, Gomes I, Kim D, Chung Y, Honig G. R., Kim K.S., Lanza R. Hemangioblastic derivatives from human induced pluripotent stem cells exhibit limited expansion and early senescence. Stem Cells. 2010;28:704–712. doi: 10.1002/stem.321. [DOI] [PubMed] [Google Scholar]
- Ferreira L.S., Gerecht S, Shieh H. F., Watson N, Rupnick M. A., Dallabrida S.M., Vunjak-Novakovic G, Langer R. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- Forsythe J.A., Jiang B. H., Iyer N.V., Agani F, Leung S. W., Koos G.L., Semenza R.D. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmiche M.C., Koteliansky V. E., Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- Gerecht-Nir S, Ziskind S, Cohen A, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–1820. doi: 10.1097/01.lab.0000106502.41391.f0. [DOI] [PubMed] [Google Scholar]
- Goldman S.A., Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner G.C., Callaghan M. J., Longaker M.T. Progress and potential for regenerative medicine. Annu Rev Med. 2007;58:299–312. doi: 10.1146/annurev.med.58.082405.095329. [DOI] [PubMed] [Google Scholar]
- Hirschi K.K., Ingram D. A., Yoder M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.K. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Kalka-Moll W. M., Silver M, Kearney M, Li T, Isner J.M., Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M.A., Hirschi K.K. Signaling hierarchy regulating human endothelial cell development. Arterioscler Thromb Vasc Biol. 2009;29:718–724. doi: 10.1161/ATVBAHA.109.184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Shizuru J. A., Uchida. N, Tsukamoto A, Weissman I.L. Toward regenerative medicine. Immunity. 2001;14:425–436. doi: 10.1016/s1074-7613(01)00123-6. [DOI] [PubMed] [Google Scholar]
- Leconte I, Fox J. C., Baldwin H. S., Buck J.L., Swain C. A. Adenoviral-mediated expression of antisense RNA to fibroblast growth factors disrupts murine vascular development. Dev Dyn. 1998;213:421–430. doi: 10.1002/(SICI)1097-0177(199812)213:4<421::AID-AJA7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Kim J. H., Lee H.J., Jeon K, Lim H, Choi H. y., Lee E.R., Park S. H., Park J.Y., Hong S, Kim S, Cho S.G. The generation of iPS cells using non-viral magnetic nanoparticlebased transfection. Biomaterials. 2011;32:6683–6691. doi: 10.1016/j.biomaterials.2011.05.070. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub J. S., Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Rouwkema J, Macdonald M, Garfein E. S., Kohane D.S., Darland D. C., Marini R, van Blitterswijk C. A., Mulligan R.C., D’Amore P.A., Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson R. E., Schulze E.N., Song H, Hsieh C. L., Pera M.F., Ying Q.L. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M.P., Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Majka S.M., Jackson K. A., Kienstra K.A., Majesky M. W., Goodell K.K., Hirschi M.A. Distinct populations of vascular progenitors in skeletal muscle are bone marrow-derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.K., Park H, Choi H. J., Kim Y, Pyun B. J., Agrawal V, Song B. W., Jeon J, Maeng Y. S., Rho S.S., Shim S, Chai J. H., Koo B.K., Hong H. J., Yun C.O., Choi C, Kim Y. M., Hwang Y.G., Kwon K.C. The WNT antagonist Dickkopf2 promotes angiogenesis in rodent and human endothelial cells. J Clin Invest. 2011;121:1882–1893. doi: 10.1172/JCI42556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat N, Garreta E, Gonzalez F, Gutierrez J, Eguizabal C, Ramos V, Borros S, Izpisua Belmonte J.C. Simple generation of human induced pluripotent stem cells using poly-beta-amino esters as the non-viral gene delivery system. J Biol Chem. 2011;286:12417–12428. doi: 10.1074/jbc.M110.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., Weissman I.L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara T, Ide Y. Tooth regeneration: implications for the use of bioengineered organs in first-wave organ replacement. Hum Cell. 2007;20:63–70. doi: 10.1111/j.1749-0774.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Narsinh K.H., Sun N, Sanchez-Freire V, Lee A. S., Almeida P, Hu S, Jan T, Wilson K. D., Leong D, Rosenberg J, Yao M, Robbins R. C., Wu J.C. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121:1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklason L.E., Gao J, Abbott W. M., Hirschi K.K., Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Putnam A.J., Mooney D.J. Tissue engineering using synthetic extracellular matrices. Nat Med. 1996;2:824–826. doi: 10.1038/nm0796-824. [DOI] [PubMed] [Google Scholar]
- Quint C, Kondo Y, Manson R. J., Lawson J.H., Dardik A, Niklason L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- Rossig L, Urbich C, Bruhl T, Dernbach E, Heeschen C, Chavakis E, Sasaki K, Aicher D, Diehl F, Seeger F, Potente M, Aicher A, Zanetta L, Dejana E, Zeiher A.M., Dimmeler S. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J Exp Med. 2005;201:1825–1835. doi: 10.1084/jem.20042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G.L. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Shin H, Jo S, Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- Sills T.M., Hirschi K.K., The emergency of blood and blood vessels in the embryo and its relevance to postnatal biology and disease . Biophysical Regulation of Vascular Differentiation and Assembly. In: Gerecht S., editor. New York: Springer; pp. pp 1–16. [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J, Shapiro S. S., Waknitz M.A., Swiergiel J. J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tulloch N.L., Muskheli V, Razumova M. V., Korte F.S., Regnier M, Hauch K. D., Pabon L, Reinecke H, Murry C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanik M.A., Bork J. A., Thomson I.I., Slukvin J.A. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- Woltjen K, Michael I. P., Mohseni. P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung H. K., Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik M. A., Smuga-Otto. K, Antosiewicz-Bourget J, Frane J. L., Tian S, Nie J, Jonsdottir G. A., Ruotti V, Stewart R, Slukvin I. I., Thomson J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ito W. D., Hopfner. U, Böhmert B, Kremer M, Reckhenrich A. K., Harder Y, Lund N, Kruse C, Machens H. G., Egaña J.T. The role of single cell derived vascular resident endothelial progenitor cells in the enhancement of vascularization in scaffold-based skin regeneration. Biomaterials. 2011;32:4109–4117. doi: 10.1016/j.biomaterials.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Zuk P.A., Zhu M, Mizuno H, Huang J, Futrell J. W., Katz A.J., Benhaim P, Lorenz H. P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]