Table 2.
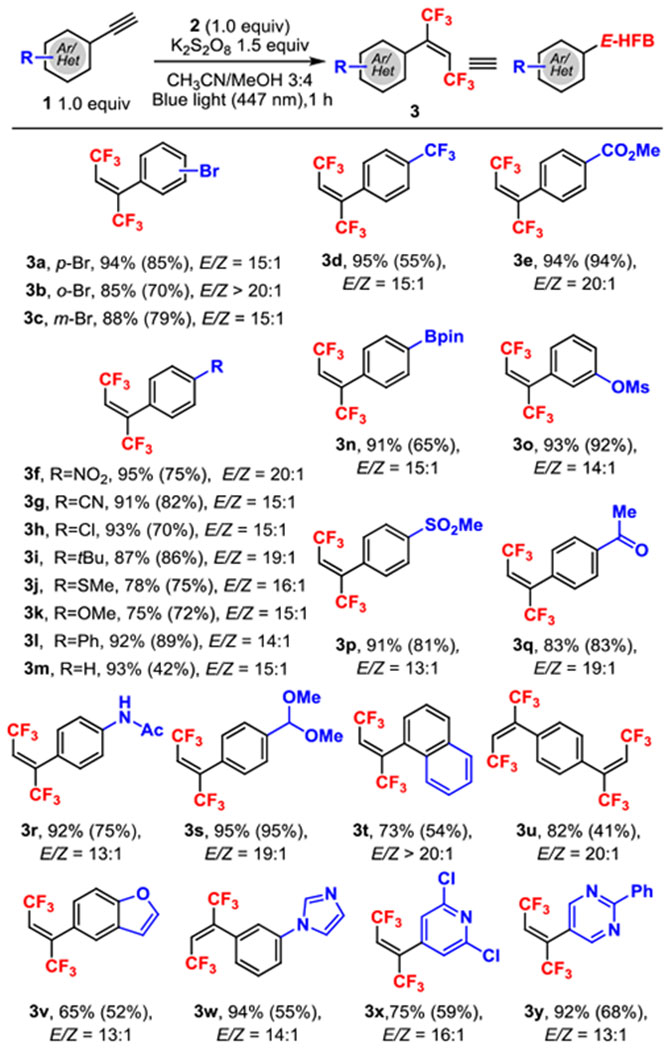 |
All reactions were with 1 (0.16 mmol) and 2 (0.16 mmol) in 2.8 mL of solvent for 1 h.
The 19F NMR yield determined by 19F NMR spectroscopy with 1-fluoro-4-methylbenzene as the internal standard, with the isolated yields given in parentheses. The E/Z ratios were determined by 19F NMR spectroscopy of the crude product mixtures.
