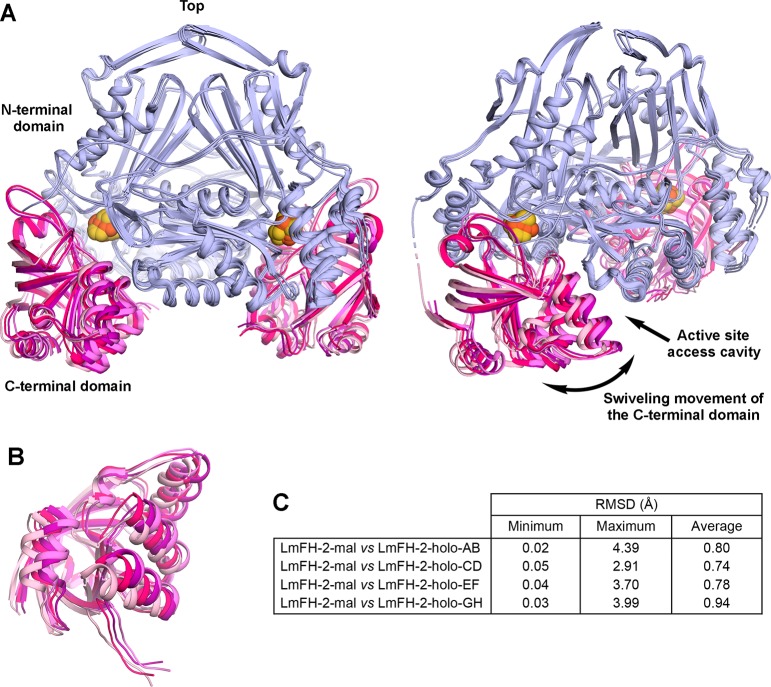
Figure 5.
Conformationally flexible LmFH-2 C-terminal domain. (A) Two views of the superposition of LmFH-2-holo dimers (chains A and B, C and D, E and F, and G and H) showing the C-terminal domain mobility. The N-terminal domain is colored light blue, and the C-terminal domain of each dimer is colored light pink (chains A and B), hot pink (chains C and D), purple (chains E and F), and violet (chains G and H). The [4Fe-4S] cluster is shown as orange (Fe) and yellow (S) spheres. (B) Another view of the LmFH-2-holo C-terminal domains showing its swiveling movement. (C) Table of RMSD values between LmFH-2 with the S-malate dimer and each LmFH-2-holo dimer. The Cα RMSD values were calculated using the ColorByRMSD PyMol script.