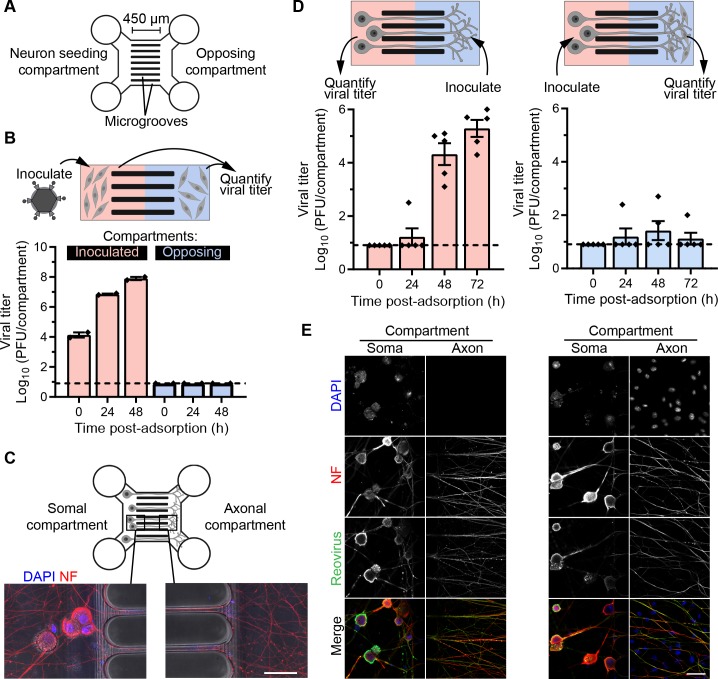
Fig 2. Anterograde spread of reovirus is limited in DRGNs.
(A) Schematic of a microfluidic device with two compartments connected by 450-μm-long microgrooves. (B) L929 cells were cultivated in both compartments (red and blue) of the microfluidic device. Cells in the left compartment were adsorbed with T3SA+ virions at an MOI of 10 PFU/cell, and viral titers in culture supernatants from both inoculated and opposing compartments were determined at the indicated times post-adsorption. Bars indicate mean titers of duplicate devices from one representative experiment. Error bars indicate SEM. (C) Representative micrographs show somal and axonal compartments of a microfluidic device with DRGNs cultivated for 7 days and stained with markers for nuclei (DAPI) and axons (non-phosphorylated neurofilament H, NF). (D-E) DRGNs cultivated in microfluidic devices were adsorbed with T3SA+ virions in the somal or axonal compartment as indicated in the schematics (D, top). L929 cells were cultivated in the axonal compartment to amplify virus released by anterograde spread following inoculation of the somal compartment. Viral titers in the culture supernatant in the compartment opposing the inoculated compartment at the indicated times post-adsorption are shown (D). Bars indicate means, and error bars indicate SEM. Individual data points represent titers from five devices in total from three independent experiments. Devices were fixed at 72 h post-adsorption and immunostained with reovirus antiserum and a neuronal marker (NF). Representative micrographs of somal and axonal compartments corresponding to the experimental setup in (D) are shown in (E). Scale bars, 50 μm. In B and D, dashed lines mark the limit of detection.