Fig. 1. Domain architecture of YART and YGT and analysis of the translocation and protease domains.
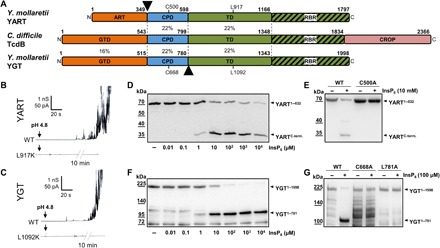
(A) Domain comparison of YART, TcdB, and YGT. The N-terminal domain of YART is an ADP-ribosyltransferase (ART), whereas the N terminus of YGT and TcdB harbors glycosyltransferases (GTD). Like TcdB, YART and YGT have an autocatalytic cysteine protease domain (CPD) and a translocation domain (TD). The C terminus of the TD of TcdB contains a receptor-binding region (RBR). Only TcdB has a CROP domain. Sequence identities of regions indicated by amino acid numbers are in percentage. Arrowheads mark split products of YART and YGT. Cys500 and Leu917 of YART and Cys668 and Leu1092 of YGT indicate the essential cysteine of CPDs and the critical leucine of TDs. (B and C) Membrane activity in lipid bilayer by YART, YGT, and their mutants YART L917K and YGT L1092K. Pore formation was induced by acidification to pH 4.5. The mutants did not induce increase in electric conductance, even after prolonged incubation (10 min). (D and F) In vitro processing of YART1–632 and YGT1–1998 at the indicated concentrations of InsP6, resulting in fragments YARTC-term and YGT1–781. (E and G) Inhibition of autocatalytic cleavage in mutants YGT1–1998 L781A, YGT1–1998 C668A, and YART1–632 C500A in the presence of InsP6. Western blots with anti-His antibody are shown. WT, wild type.
