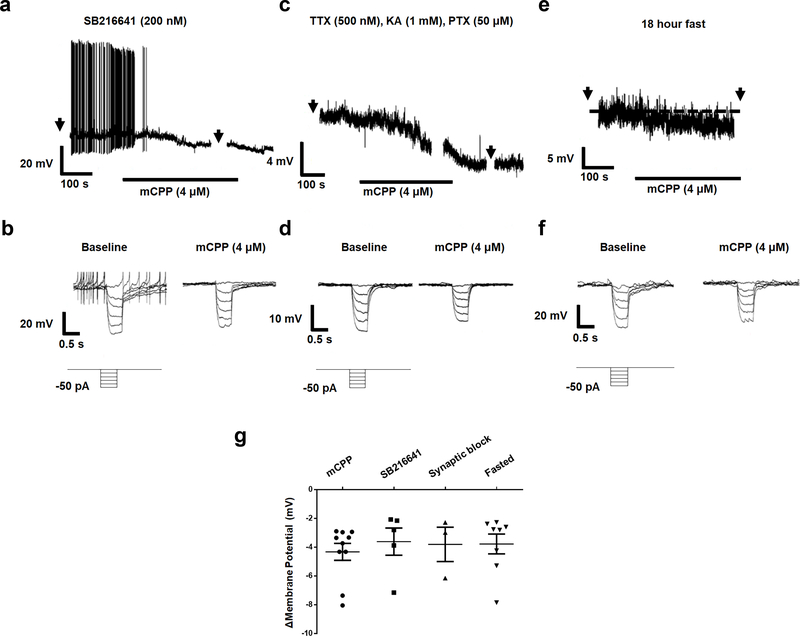
Extended Data Fig. 9: Hyperpolarisation of LPBNHtr2c neurons is not dependent on Htr1b and is postsynaptic.
(a) Representative current clamp recording of an LPBNHtr2c neuron pre-treated with SB216641 (200 nM). Hyperpolarisation in response to mCPP persists in presence of SB216641. Arrows indicate time at which current steps were applied. (b) Voltage deflections in response to hyperpolarising currents from the same neuron, showing decreased input resistance in response to mCPP. Current steps in trace made in 10 pA increments from −50 pA to 0 pA. (c) Representative current clamp recording of an LPBNHtr2c neuron pre-treated with TTX (500 nM), kynurenic acid (KA, 1 mM) and picrotoxin (PTX, 50 μM), showing hyperpolarisation in response to mCPP. Arrows indicate time at which current steps were applied. (d) Voltage deflections in response to hyperpolarising currents from the same neuron, showing decreased input resistance in response to mCPP. Current steps in trace made in 10 pA increments from −50 pA to 0 pA. (e) Representative current clamp recording of an LPBNHtr2c neuron from a fasted mouse (18 hours), showing hyperpolarisation in response to mCPP. Arrows indicate time at which current steps were applied. (f) Voltage deflections in response to hyperpolarising currents from the same neuron, showing decreased input resistance in response to mCPP. Current steps in trace made in 10 pA increments from −50 pA to 0 pA. (g) Graph summarising change in membrane potential in response to mCPP under conditions tested. Neither pre-treatment with SB216641, Synaptic blockers nor recordings from fasted mice changed the magnitude of response to mCPP. mCPP n = 10 cells, SB216641 n = 5 cells, Synaptic block n = 3 cells, Fasted n = 8 cells. Data represented as mean ± s.e.m