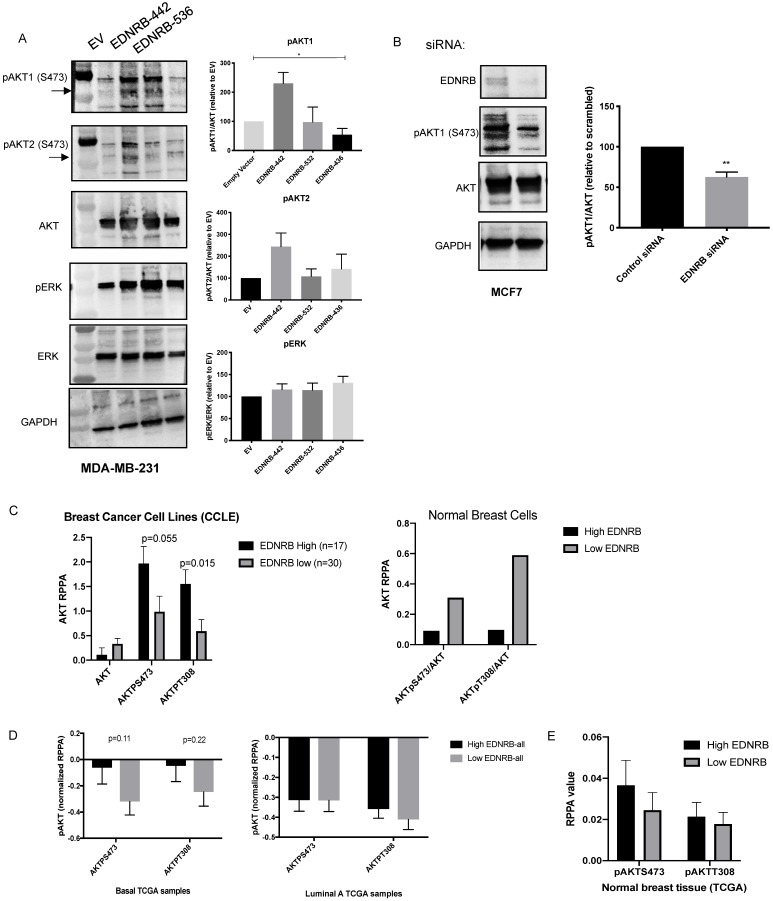
Figure 4.
EDNRB isoforms differentially regulate cellular signaling. Western blotting of lysates extracted from (A) MDA-MB-231 cells transfected with EDNRB isoforms and (B) MCF-7 cells transfected EDNRB-specific siRNA were probed with antibodies specific for phosphorylated AKT1 and phosphorylated AKT2 at S473 (pAKT1, pAKT2), pan AKT, phosphorylated ERK (pERK), pan ERK, and GAPDH. Bands were quantitated using ImageJ Software and normalized to GAPDH intensity. (A) MDA-MB-231 cells transfected with EDNRB-442 had significantly higher levels of pAKT1 (ANOVA p=0.013) but not pAKT2 (p=0.28) or pERK (p=0.42); (B) MCF-7 cells transfected with EDNRB-siRNA had significantly reduced pAKT1/AKT levels (p=0.097). (C) Analysis of breast cancer cell lines from Cancer Cell Line Encyclopedia data (CCLE) shows a non-significant positive association between EDNRB and active AKT (left); this trend was not observed in non-transformed breast cell lines (right). (D) TCGA breast cancer exon expression data was separated by subtype and analyzed by median EDNRB expression using two isoform-specific probes that recognize EDNRB-442 or EDNRB-532; pAKT levels at both S473 and T308 sites from the RPPA dataset were compared between EDNRB-high and low groups (n=60 for both groups) in basal and (left) luminal A (right) breast cancer subtypes, and (E) normal breast tissue.