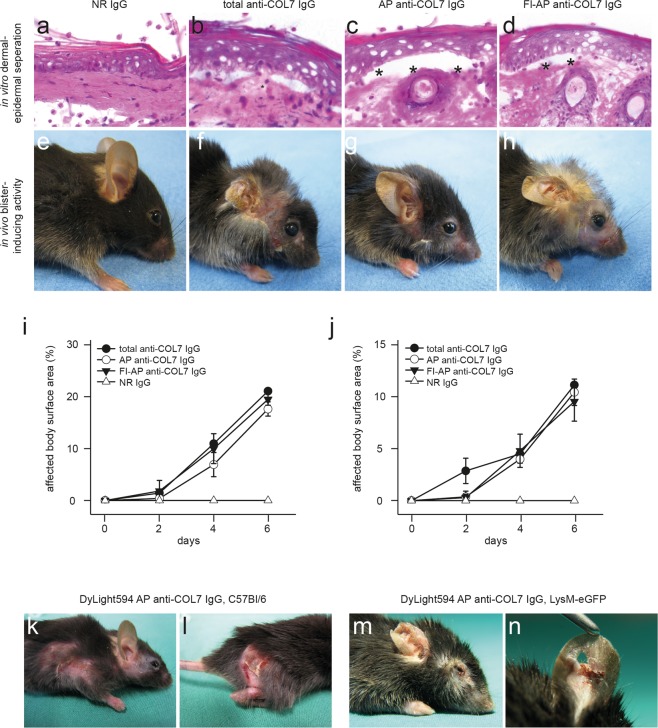
Figure 1.
Pathogenicity of fluorescently-labeled, affinity-purified anti-COL7 IgG. (a–d) Sections of normal mouse skin were incubated with (a) normal rabbit IgG, (b) IgG purified form rabbits immunized with COL7, (c) affinity-purified (AP) anti-COL7 IgG, or (d) DyLight488 fluorescently-labeled (Fl), AP anti-COL7 IgG, followed by administration of peripheral polymorphnuclear cells. Similar concentrations of anti-COL7 IgG were added to conditions b-d, which induced ex vivo dermal-epidermal separation under all experimental conditions. (e–h) C57Bl/6 mice were s.c. injected with the indicated IgG preparations. Amount of anti-COL7 IgG was identical in conditions f-h, and induced a comparable extend of skin blistering, as demonstrated for immune preparations. (i) SA6307 and (j) SA6306. Data in i-j is based on 3-4 mice per group. (k,l) DyLight594-labelled AP anti-COL7 IgG was s.c. injected into a total of 3 C57Bl/6 mice. Representative clinical photographs of 2 of these mice obtained 12 days after the initial IgG injection are shown here, demonstrating extensive skin lesions. (m,n) DyLight594-labelled AP anti-COL7 IgG was s.c. injected into 3 LysM-eGFP mice. Representative clinical photographs of 2 of these mice obtained 12 days after the initial IgG injection are shown here. The data are expressed as the mean ± SEM. To compare the differences in the disease severity (AUC), independent samples Student’s t-tests were used. A p-value <0.05 was considered statistically significant.