Figure 5.
Mutations in the MERTK Gene Lead to Defects in POS Phagocytosis
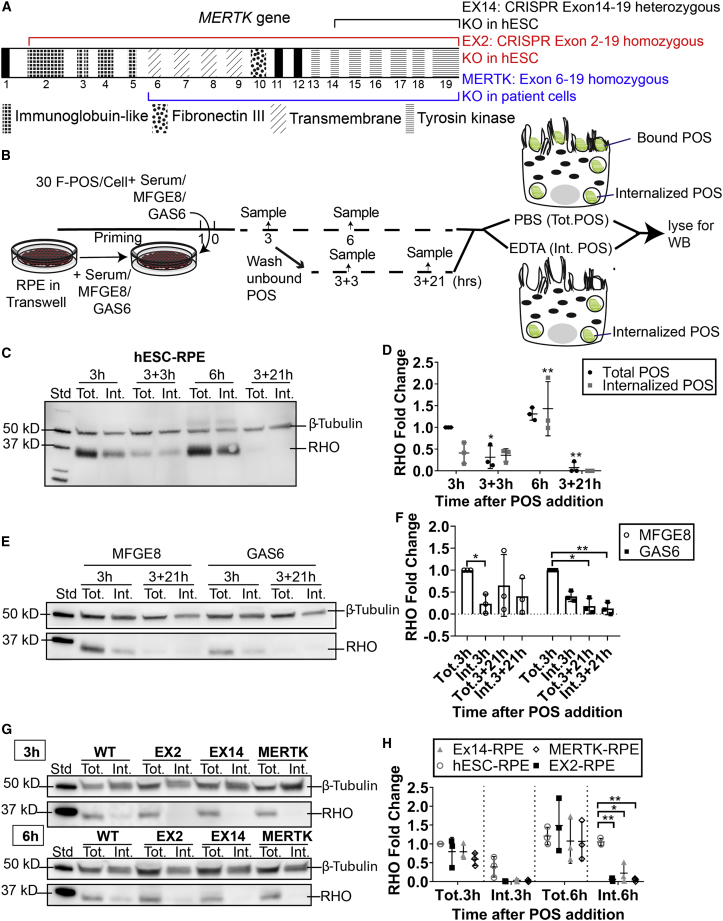
(A) An illustration of the MERTK gene showing the different exons coding for the different protein domains of the receptor. Exons are labeled 1 to 19. The mutations in the three stem cell lines used in this study are also represented in the illustration: the iPSC cell line derived from an RP38 patient has a homozygous deletion spanning exon 6 to 19 (blue). CRISPR/Cas9-mediated partial deletion of exon 14 to 19 in hESCs is shown in black. CRISPR/Cas9-mediated deletion of exon 2 to 19 in hESCs is shown in red. See also Figures S4–S7 for MERTK mutations details, patient clinical observations, stem cell characterization, and rescue in isogenic iPSC-RPE control.
(B) A schematic of the three experiments in (C–H). All samples were treated with either PBS or EDTA before lysis to detect total versus internalized F-POS, respectively. Blots were probed for β-tubulin as a loading control, and RHO to indicate total POS (Tot.) and internalized POS (Int.). RHO signal was normalized to tubulin and then to the total POS signal in control condition to monitor the fold change in RHO signal. Data are represented as means ± SD. N = 3 biological repeats. Significance was calculated using two-way ANOVA test. ns > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.
(C) HESC-RPE were primed for 1 h with 30% serum. Next, cells were challenged with F-POS and incubated for 3 or 6 h at 37°C. Samples were lysed 3 or 6 h after POS addition. Other samples were washed at 3 h and kept for 3 h (3 + 3 h) or 21 h (3 + 21 h) before lysis to monitor POS degradation.
(D) Quantification of the immunoblot in (C). Total POS 3 h was used as control condition for normalization and statistical significance calculation.
(E) HESC-RPE were primed for 1 h with either GAS6 or MFGE8. Next, cells were challenged with POS and incubated for 3 h and either lysed or washed and kept for a further 21 h (3 + 21 h) before lysis to monitor POS degradation.
(F) Quantification of the immunoblot in (E). Total POS 3 h in each condition was used as control for normalization and statistical significance calculation. HESC-RPE bind, internalize, and degrade POS efficiently in the presence of GAS6 and serum. In contrast, in the presence of MFGE8 POS degradation is slowed down. See Figure S3 for fluorescence-based POS phagocytosis data.
(G) Wild-type and MERTK mutant RPE were primed for 1 h with 30% serum. Next, cells were challenged with POS and incubated for 3 or 6 h at 37°C before processing.
(H) Quantification of the immunoblot in (G). Total POS 3 h in hESC-RPE was used as control condition for normalization. For significance calculation all samples were compared with each other. MERTK mutant RPE show POS internalization defect.