Figure 7.
Quantitative Live Imaging Analysis Shows that MERTK Mutant Human RPE Fail to Fragment and Internalize POS
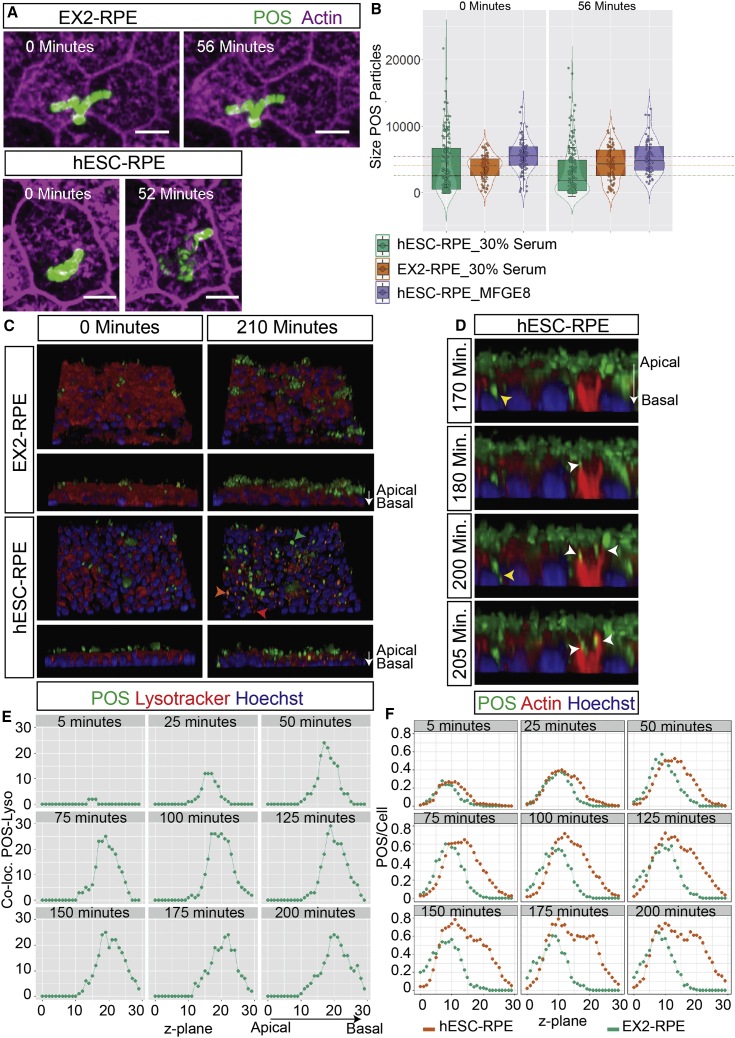
(A) Snapshots from time-lapse live fluorescence imaging performed with LSM 880 upright microscope using the Airyscan feature and a 40× dipping objective. The complete time-lapse videos can be found in Videos S1a and S1b. HESC-RPE and EX2-RPE (grown on transwells) were primed with 30% serum or 5 μg/mL MFGE8 for 1 h, and were challenged with AF488-labeled W-POS for 2.5 h at 37°C with 5% CO2 before imaging. Membranes, containing the RPE monolayer, were cut out from the Transwell insert and placed with the apical side of the cells facing the dipping objective in a 35-mm cell culture dish with 2 mL medium containing SiR-actin dye and 30% serum. (A) Many of the whole POS particles that were still seen at the start of imaging (0 min) in hESC-RPE got fragmented within around 52 min. In contrast, whole POS particles in EX2-RPE did not get fragmented within the same or longer time frame (56 min). Scale bar, 10 μm.
(B) Box-violin plot of the live imaging performed in (A) shows distribution of the POS particle size under different conditions at the start of the imaging (0 min) and at the end (56 min).
(C and D) Snapshots from time-lapse live fluorescence imaging performed with an inverted Leica confocal microscope (SP5-mp) on hESC-RPE and EX2-RPE plated in 96-well plates, and treated with 30% serum and SiR-actin (D) or LysoTracker (C), to monitor POS internalization or co-localization with lysosomes, respectively. The complete time lapse can be found in Video S2 (EX2-RPE) and Video S3 (hESC-RPE). Following priming with 30% serum W-POS were added to the cells and imaging started 25 min thereafter without a washing step in between. (C) In hESC-RPE, W-POS (green arrow) were fragmented into smaller pieces and co-localized with lysosomes (orange arrow). In contrast, W-POS remained intact in EX2-RPE and did not co-localize with lysosomes. (D) Actin labeling showed many internalized POS fragments in hESC-RPE (yellow arrow). Some POS fragments were captured during the process of internalization, which takes around 30 min (white arrow).
(E) Quantification of the number of POS that co-localize with lysosomes at different time points during the time lapse and in different z-planes, where (0) refers to the apical surface of the RPE and (30) refers to the basal. The number of co-localized POS increased with time and shifted toward the basal side of the RPE.
(F) The number of POS/cell in hESC-RPE over the different z-planes and time points during the time lapse showed a shift in the peak of POS/cell count toward basal z planes with time, reflecting internalization. This shift was not detected in EX2-RPE.