Figure 1.
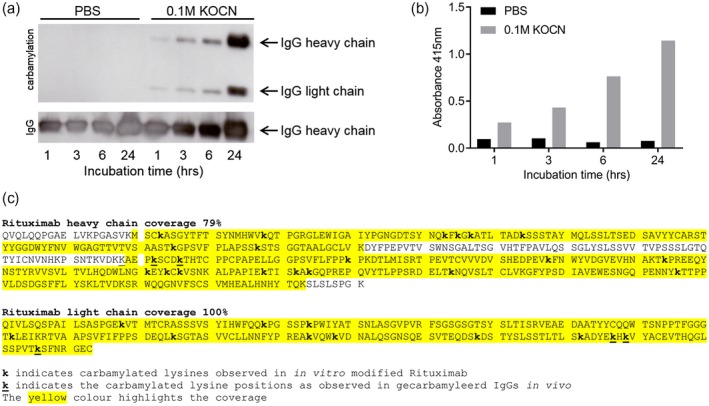
Successful carbamylation (ca) of rituximab. The results from rituximab are shown and are representative for all carbamylated antibodies [intravenous immunoglobulin [(IVIg), alemtuzumab, dinitrophenyl (DNP) variants]. Antibodies were either incubated in phosphate‐buffered saline (PBS) as a control (buffer only) or in 0·1 M potassium cyanate (KOCN). (a) Western blot analysis of 2 µg (ca)‐rituximab, carbamylation was visualized using a carbamyl–lysine‐specific antibody. Equal loading of the preparations was shown by detection of human IgG. Incubation time is indicated in hours. (b) Carbamylation of rituximab as analyzed by enzyme‐linked immunosorbent assay (ELISA), where an equal amount of antibody (10 µg/ml) was used to coat the well. Detection was performed using a carbamyl–lysine‐specific antibody. (c) Mass spectrometry analysis of rituximab that was carbamylated for 24 h. The carbamylated lysines are annotated (k), coverage is indicated with yellow highlight.
