Figure 2.
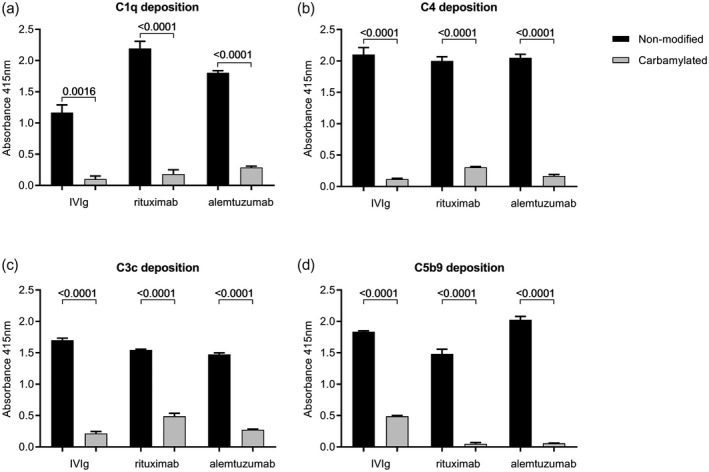
Complement deposition of C1q, C4, C3c and C5b9 on non‐modified and carbamylated immunoglobulin (Ig)G. Complement deposition enzyme‐linked immunosorbent assays (ELISAs) were performed using 10 μg/ml plate‐bound non‐modified or carbamylated (ca) intravenous immunoglobulin [(IVIg), rituximab and alemtuzumab. C1q (a), C4 (b), C3c (c) and C5b9 (d) deposition after incubation for 1 h with 1% normal human serum (NHS) was measured at an absorbance wavelength of 415 nm. Data are shown as mean with standard deviation from technical triplicate. All differences in complement deposition between non‐modified antibodies and their carbamylated counterparts were significant, as analyzed with t‐test (all P < 0·05). Shown is a representative experiment performed at least twice.
