Figure 3.
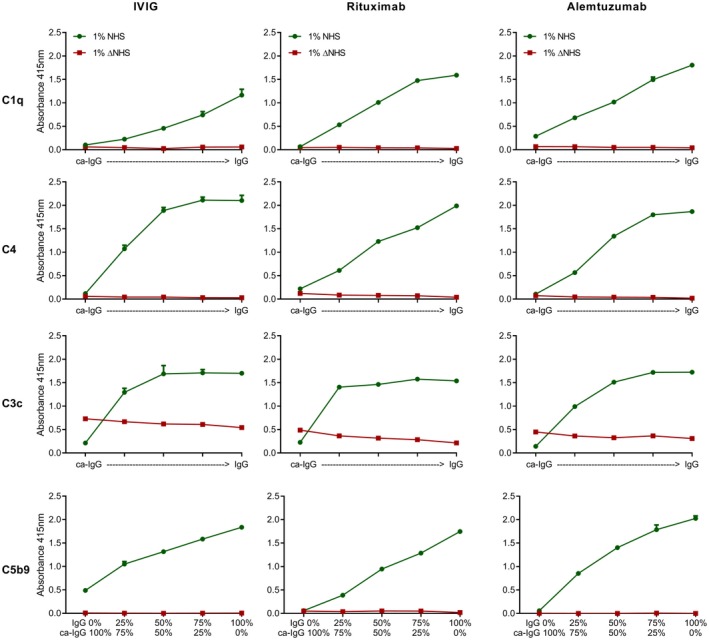
Deposition of different complement components is not affected by the presence of carbamylated (ca)‐immunoglobulin (Ig)G. Complement deposition enzyme‐linked immunosorbent assays (ELISAs) were performed using plate‐bound non‐modified or ca‐IVIg, rituximab and alemtuzumab, with a sum of 10 μg/ml coating mixed in different ratios, and for rituximab a sum of 2·5 μg/ml (C4) or 5 μg/ml (C1q, C3c, C5b9). From left to right, ca‐IgG versus non‐modified IgG: 100%: 0%, 75%: 25%, 50%: 50%, 25%: 75%, 0%: 100%. Deposition of complement components C1q, C4, C3c and C5b9 were measured at an absorbance wavelength of 415 nm. Green circles depict the complement deposition after incubation with 1% normal human serum (NHS), red squares depict complement deposition after incubation with 1% heat‐inactivated normal human serum (ΔNHS) for 1 h. Shown is a representative experiment which has been performed independently at least twice.
