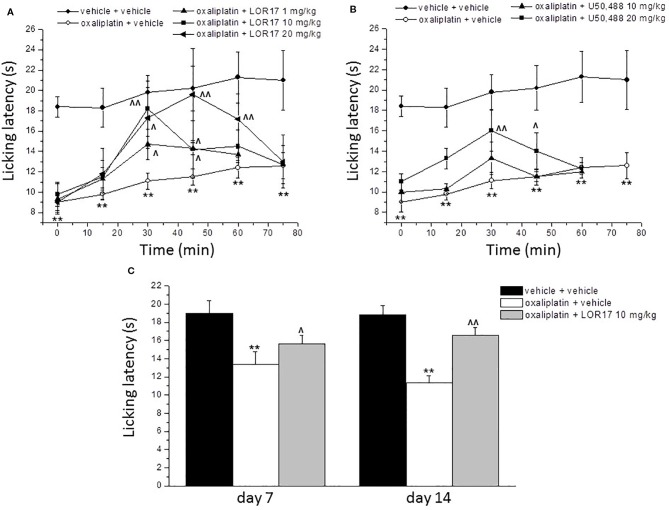
Figure 8.
Effect of LOR17 on oxaliplatin-induced neuropathic pain. (A). Acute treatment: on day 14 of oxaliplatin administration (2.4 mg/kg, i.p., administered daily), LOR17 (1 – 20 mg/kg) was administered s.c. The response to a thermal non-noxious stimulus was evaluated over time by the cold plate test measuring the latency to pain-related behaviors (lifting or licking of the paw). (B). LOR17 effects were compared with those induced by the acute administration of U50,448 (10 and 20 mg/kg, s.c.). (C). Repeated treatment: LOR17 (10 mg/kg) was administered s.c. daily starting the first day of oxaliplatin treatment. Cold hypersensitivity (cold plate test) was performed on days 7 and 14. Each value represents the mean ± S.E.M. of 12 mice performed in 2 different experimental set. **P < 0.01 versus vehicle + vehicle treated animals; ^ P < 0.05 versus oxaliplatin + vehicle; ^^ P < 0.01 versus oxaliplatin + vehicle (Bonferroni test after ANOVA).