Figure 2.
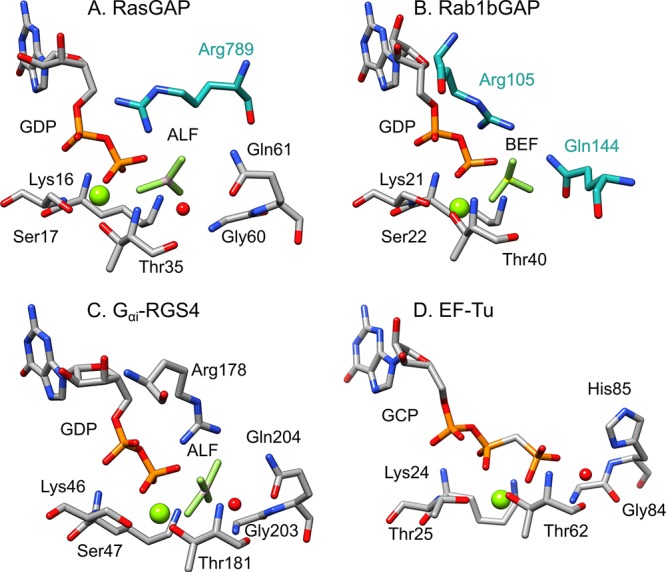
Active sites of three different signaling proteins: (A) RasGAP in complex with AlF3 (AF3) and a phosphonate GDP analogue (GNP) (PDB ID: 1WQ1(11,12)), (B) human Rab1 in complex with the GTPase activating protein (GAP) domain of TBC1D20 and BeF3– (BEF) (PDB ID: 4HLQ(12,13)), and (C) the α-subunit of a heterotrimeric G protein in complex with GDP and AlF4– (ALF) (PDB ID: 1AGR(12,14)). Shown here for comparison is also the active site of (D) Elongation Factor Thermo unstable (EF-Tu) in complex with a GTP analogue (GCP, E. coli numbering) (PDB ID: 4V5L(12,15)). Residues from each GTPase are highlighted in gray, and from the GAPs in cyan. The residues inserted by the regulatory proteins are specifically (A) the arginine finger in the RasGAP complex, (B) the arginine and glutamine fingers in the RabGAP complex. In case (C), the arginine (Arg178) and glutamine (Gln204) are intrinsic to the GTPase itself, and the catalytic stimulation by the regulatory protein is thus entirely allosteric through a so-called “dual-finger mechanism”. Adapted with permission from ref (7) (direct link: https://pubs.acs.org/doi/10.1021/jacs.9b03193). Copyright 2019 American Chemical Society.
