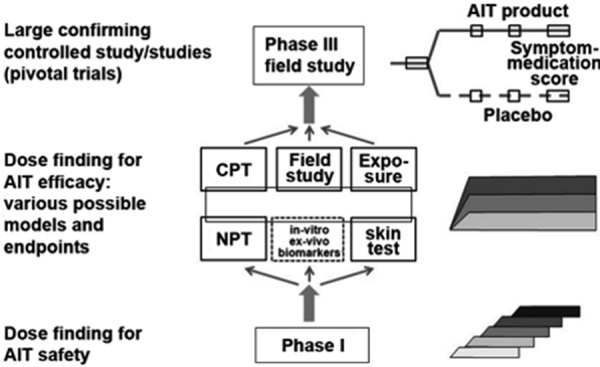
Figure 1. Clinical development program for allergen immunotherapy. Center: Possible models and methods for phase II dose finding studies on efficacy. Additional laboratory parameters or biomarkers (dotted box) are optional but not sufficient as study endpoints. AIT = allergen immunotherapy; CPT = conjunctival provocation test; NPT = nasal provocation test; Exposure = provocation in exposure chamber.