Table 2.
Summary of treatment‐emergent adverse events in cycle 1 and throughout the study (safety population)
| Patients with: | Cycle 1 | Entire study | ||
|---|---|---|---|---|
| IV NEPA (n = 200), n (%) | Oral NEPA (n = 202), n (%) | IV NEPA (n = 200), n (%) | Oral NEPA (n = 203),a n (%) | |
| At least one treatment‐emergent adverse event (TEAE) | 121 (60.5) | 122 (60.4) | 184 (92.0) | 187 (92.1) |
| Severe (grade ≥3) TEAEs | 11 (5.5) | 10 (5.0) | 37 (18.5) | 29 (14.3) |
| Serious TEAE | 2 (1.0) | 1 (0.5) | 5 (2.5) | 4 (2.0) |
| Any treatment‐related adverse event (TRAE) | 13 (6.5) | 12 (5.9) | 16 (8.0) | 22 (10.8) |
| Severe (grade ≥3) TRAE | 0 | 0 | 1 (0.5) | 2 (1.0) |
| Serious TRAE | 0 | 0 | 0 | 1 (0.5)b |
| Any TEAE leading to discontinuation | 0 | 0 | 1 (0.5) | 0 |
| Any TRAE leading to discontinuation | 0 | 0 | 0 | 0 |
| Any TEAE resulting in death | 0 | 0 | 0 | 0 |
| Any infusion‐site TEAE | 2 (1.0) | 0 | 3 (1.5) | 9 (4.4) |
| Any treatment‐related infusion site TEAE | 0 | 0 | 0 | 0 |
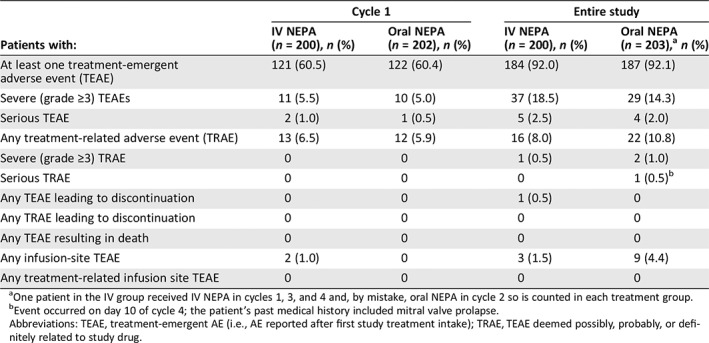
One patient in the IV group received IV NEPA in cycles 1, 3, and 4 and, by mistake, oral NEPA in cycle 2 so is counted in each treatment group.
Event occurred on day 10 of cycle 4; the patient's past medical history included mitral valve prolapse.
Abbreviations: TEAE, treatment‐emergent AE (i.e., AE reported after first study treatment intake); TRAE, TEAE deemed possibly, probably, or definitely related to study drug.
