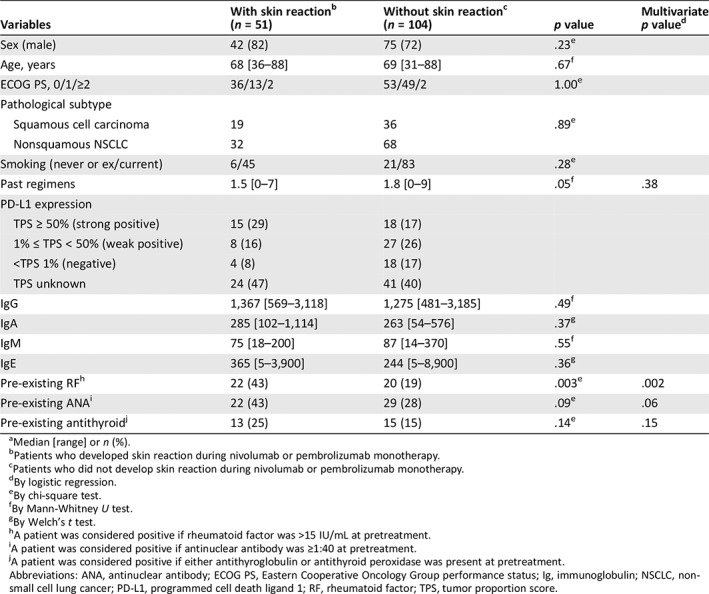
Table 3.
Characteristics of patients with or without skin reaction during nivolumab or pembrolizumab monotherapy (n = 155)a
| With skin reactionb | Without skin reactionc | Multivariate | ||
|---|---|---|---|---|
| Variables | (n = 51) | (n = 104) | p value | p valued |
| Sex (male) | 42 (82) | 75 (72) | .23e | |
| Age, years | 68 [36–88] | 69 [31–88] | .67f | |
| ECOG PS, 0/1/≥2 | 36/13/2 | 53/49/2 | 1.00e | |
| Pathological subtype | ||||
| Squamous cell carcinoma | 19 | 36 | .89e | |
| Nonsquamous NSCLC | 32 | 68 | ||
| Smoking (never or ex/current) | 6/45 | 21/83 | .28e | |
| Past regimens | 1.5 [0–7] | 1.8 [0–9] | .05f | .38 |
| PD‐L1 expression | ||||
| TPS ≥ 50% (strong positive) | 15 (29) | 18 (17) | ||
| 1% ≤ TPS < 50% (weak positive) | 8 (16) | 27 (26) | ||
| <TPS 1% (negative) | 4 (8) | 18 (17) | ||
| TPS unknown | 24 (47) | 41 (40) | ||
| IgG | 1,367 [569–3,118] | 1,275 [481–3,185] | .49f | |
| IgA | 285 [102–1,114] | 263 [54–576] | .37g | |
| IgM | 75 [18–200] | 87 [14–370] | .55f | |
| IgE | 365 [5–3,900] | 244 [5–8,900] | .36g | |
| Pre‐existing RFh | 22 (43) | 20 (19) | .003e | .002 |
| Pre‐existing ANAi | 22 (43) | 29 (28) | .09e | .06 |
| Pre‐existing antithyroidj | 13 (25) | 15 (15) | .14e | .15 |
Median [range] or n (%).
Patients who developed skin reaction during nivolumab or pembrolizumab monotherapy.
Patients who did not develop skin reaction during nivolumab or pembrolizumab monotherapy.
By logistic regression.
By chi‐square test.
By Mann‐Whitney U test.
By Welch's t test.
A patient was considered positive if rheumatoid factor was >15 IU/mL at pretreatment.
A patient was considered positive if antinuclear antibody was ≥1:40 at pretreatment.
A patient was considered positive if either antithyroglobulin or antithyroid peroxidase was present at pretreatment.
Abbreviations: ANA, antinuclear antibody; ECOG PS, Eastern Cooperative Oncology Group performance status; Ig, immunoglobulin; NSCLC, non‐small cell lung cancer; PD‐L1, programmed cell death ligand 1; RF, rheumatoid factor; TPS, tumor proportion score.
